Chapter 6, Part 6
advertisement
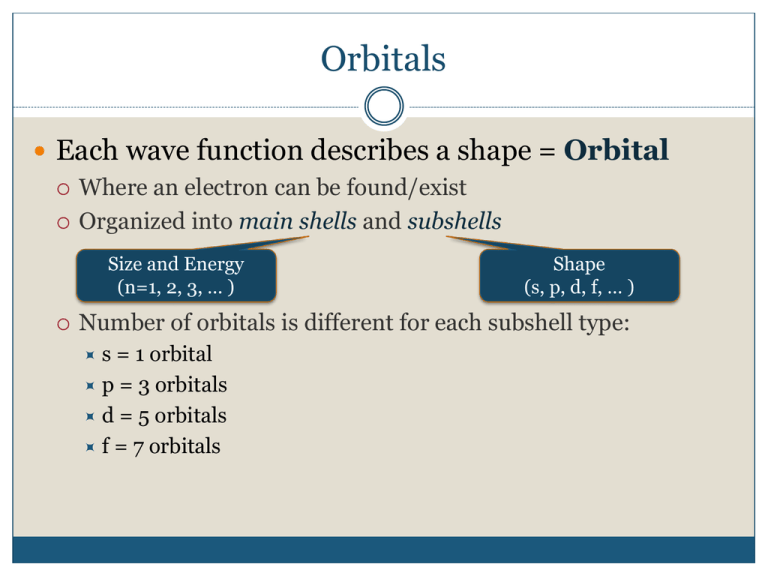
Orbitals Each wave function describes a shape = Orbital Where an electron can be found/exist Organized into main shells and subshells Size and Energy (n=1, 2, 3, … ) Shape (s, p, d, f, … ) Number of orbitals is different for each subshell type: s = 1 orbital p = 3 orbitals d = 5 orbitals f = 7 orbitals “Allowed” Orbitals The probability density can only take certain shapes at each n energy level, or main shell 4 3 ENERGY 2 1 4f ___ ___ ___ ___ ___ ___ ___ 4d ___ ___ ___ ___ ___ 4p ___ ___ ___ 3d ___ ___ ___ ___ ___ 4s ___ 3p ___ ___ ___ 3s ___ 2p ___ ___ ___ 2s ___ •1, 2, and 3 correspond to the major energy levels (main shells) 1s ___ •At the same main shell level, a p orbital will be at a higher energy than an s orbital What type of orbital is this? 1. s 56% 2. pz 36% 3. dxy 4. dxz 5% 1 3% 2 3 4 Which type of orbital can’t exist? 1. 1px 25% 25% 25% 2 3 25% 2. 2px 3. 2s 4. 3dxy 1 4 10 NODES- Where electrons don’t go Spherical Nodes Hydrogen You too can play with hydrogen… http://homepages.ius.edu/kforinas/physlets/quantum/hydrogen.html Quantum # Rules There are four different quantum numbers: n, l, ml, and ms n, l, and ml are integers n cannot be zero l can be 0 to n-1 ml can be anything from –l to l ms can be +½ or -½ Quantum Numbers and Orbitals Nodes, Revisited # of planar nodes = l # of spherical nodes = n – l – 1 Total # nodes = n – 1 Example: 3d orbital What orbital has these quantum numbers? n = 3, l = 2, ml = -1 1. 4p 20% 20% 20% 2 3 20% 20% 2. 3d 3. 3p 4. 1d 5. 2f 1 4 5 10 What are the quantum numbers for the 5dxy orbital? 1. n = 5, l = 2, ml = 0 20% 20% 20% 2 3 20% 20% 2. n = 5, l = -2, ml = 3 3. n = 4, l = 2, ml = 2 4. n = 5, l = 3, ml = 0 5. n = 5, l = 2, ml = -5 1 4 5 10 Which is not a valid set of quantum numbers? 1. n = 4, l = 1, ml = -1 20% 20% 20% 2 3 20% 20% 2. n = 1, l = 0, ml = 0 3. n = 6, l = 5, ml = -5 4. n = 2, l = 2, ml = 1 5. n = 3, l = 2, ml = 2 1 4 5 10 Rules for filling orbitals 1. Pauli Exclusion Principle No two electrons can have the same 4 quantum numbers An orbital has a maximum of 2 electrons of opposite spin 2. Aufbau/Build-up Principle Lower energy levels fill before higher energy levels 3. Hund’s Rule Electrons only pair after all orbitals at an energy level have 1 electron 4. Madelung’s Rule Orbitals fill in the order of the value of n + l Orbital Filling Order 4 3 ENERGY 2 1 4f ___ ___ ___ ___ ___ ___ ___ 4d ___ ___ ___ ___ ___ 4p ___ ___ ___ 3d ___ ___ ___ ___ ___ 4s ___ 3p ___ ___ ___ 3s ___ 2p ___ ___ ___ 2s ___ •1, 2, and 3 correspond to the major energy levels (main shells) 1s ___ •At the same main shell level, a p orbital will be at a higher energy than an s orbital








