2.1 The mole concept
advertisement

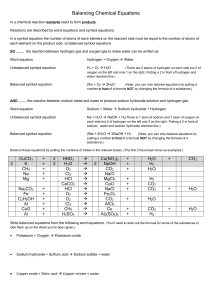
2.1 The mole concept Essential idea The mole makes it possible to correlate the number of particles with the mass that can be measured. Understandings You should know that 1. Molar mass (M) has the unit gmol-1 2. The number of significant figures in a result is based on the figures given in the data. Applications and skills You should be able to 1. Calculate the molar masses of molecules and formula units. 2. Solve problems involving the relationships between the amount of substance in moles and the mass in grams. Concept Check 1. Camels store the fat tristearin, C57H110O6 in their humps. The fat reacts with the oxygen from respiration to produce water, which the camel stores in its hump for later use. Calculate the mass of water produced from 1000. g of fat? 2 C57H110O6 + 163 O2 → 114 CO2 + 110 H2O 2. Lithium oxide is used on board a space shuttle to remove water from the atmosphere according to the equation: Li2O + H2 O → LiOH Calculate the mass of lithium oxide that is needed on a shuttle to remove 80100g of water? 3. Consider the following equation for the process of rusting: Fe + O2 + H2O → Fe2O3.H2O a) If a piece of iron of mass 25.0 g rusts away to form iron (III) oxide monohydrate, calculate the mass of rust would be formed? b) What does the . refer to in Fe2O3.H2O and what is type of compound called? 4. Soda lime glass is prepared by melting sodium carbonate, Na2CO3 and sand, SiO2. The composition of glass varies, but the generally accepted formula is Na2O.CaO.6SiO2. Find the mass of sand in grams and kilograms that would be required to produce 4.00 x 102g of glass? Na2CO3 + CaCO3 + 6 SiO2 → Na2O.CaO.6SiO2 (Soda-lime glass) + 2 CO2 5. The reaction involved in baking powder (a mixture of potassium hydrogen tartrate and sodium hydrogen carbonate) is: KHC4H4O6 + NaHCO3 → KNaC4H4O6 + H2 O + CO2 A recipe for banana cake calls for two level teaspoons (8.0g) of potassium hydrogen tartrate. Determine the mass of sodium hydrogen carbonate that must be added for both materials to react completely? 6. In 1938, the British Interplanetary Society suggested the use of sodium hydroxide, to remove carbon dioxide produced by the astronauts in their exhaled air from the cabin atmosphere during an expedition to the moon. The average human body discharges approximately 938g of carbon dioxide per day. Determine the mass of sodium hydroxide that must be carried on board to absorb all this carbon dioxide for a ten day voyage by one astronaut? CO2 (g) → NaOH (s) + NaHCO3 (s) Peer Assessment: Have you made your thinking visible? To what extent did your peers make their thinking visible? Assess their problem solving process in the following way. 2: Complete 1: Partially complete. An attempt has been made but there aspects missing or incorrect. 0: Not done at all Allow errors to be carried forward. Check the box that applies Criteria 1 A balanced equation for the reaction is shown 2 For each calculation a relevant formula(s) with the quantity and subject is given. E.g. m(NaCl) = n x M 3 For each calculation the values have been substituted into the formula. 4 For each calculation the answer has the correct unit and number of significant figures. 5 Mole ratios are clearly shown 6 The final answer is calculated correctly 7 The final answer has the correct number of significant figures 8 The final answer has the correct units 2 1 0 Comments: If you awarded ‘partial’ or ‘not at all’ for any aspect please provide justification. Total out of 16 points =