Identifying Chemical Compounds Worksheet
advertisement
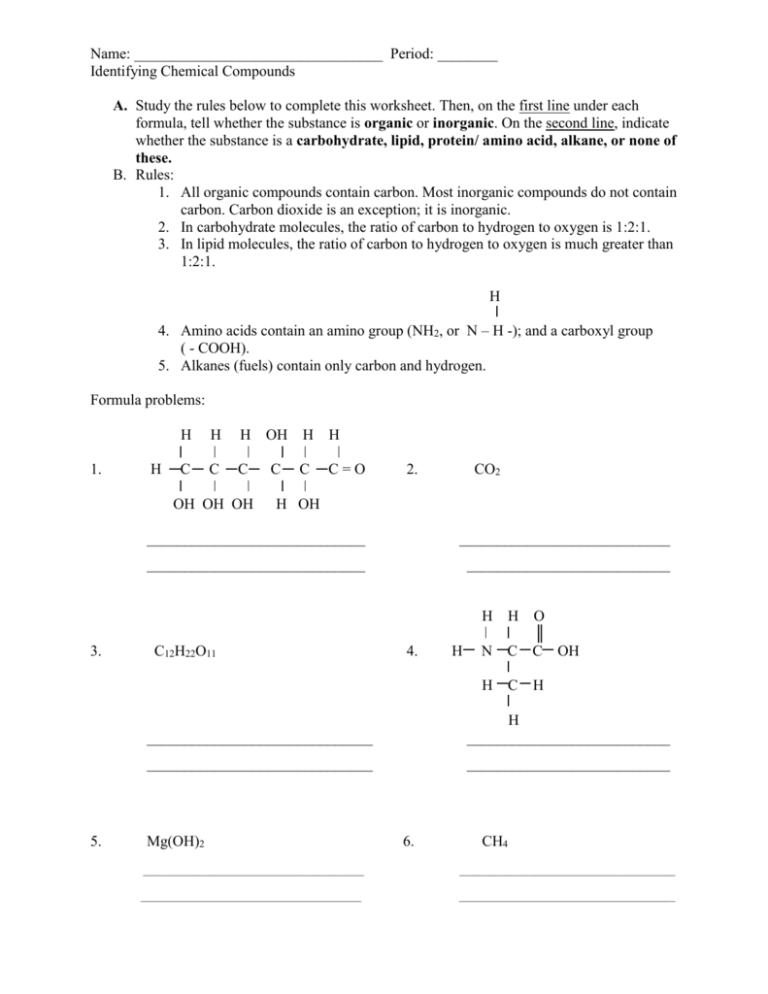
Name: _________________________________ Period: ________ Identifying Chemical Compounds A. Study the rules below to complete this worksheet. Then, on the first line under each formula, tell whether the substance is organic or inorganic. On the second line, indicate whether the substance is a carbohydrate, lipid, protein/ amino acid, alkane, or none of these. B. Rules: 1. All organic compounds contain carbon. Most inorganic compounds do not contain carbon. Carbon dioxide is an exception; it is inorganic. 2. In carbohydrate molecules, the ratio of carbon to hydrogen to oxygen is 1:2:1. 3. In lipid molecules, the ratio of carbon to hydrogen to oxygen is much greater than 1:2:1. H 4. Amino acids contain an amino group (NH2, or N – H -); and a carboxyl group ( - COOH). 5. Alkanes (fuels) contain only carbon and hydrogen. Formula problems: 1. H H H H OH H H C C C C C C=O OH OH OH 2. CO2 H OH _____________________________ ____________________________ _____________________________ ___________________________ H 3. C12H22O11 4. H H O ║ N C C OH H 5. C H ______________________________ H ___________________________ ______________________________ ___________________________ Mg(OH)2 6. CH4 ____________________________________________ ___________________________________________ ____________________________________________ ___________________________________________ 7. C18H34O3 8. CH3COOH ______________________________ ___________________________ ______________________________ ___________________________ 9. 10. ______________________________ ___________________________ ______________________________ ___________________________ 11. 12. NaCl ______________________________ ______________________________ ___________________________ ___________________________ 13. C3 H5(C17H35O2)3 ______________________________ ______________________________ On the line provided, identify the common name for these compounds. 14. Sodium hydrogen carbonate _______________ 15. Methane ______________________ 16. Solid carbon dioxide _____________________ 17. Ascorbic acid __________________ 18. Sucrose _______________________________ 19. Acetic acid ___________________ 20. Calcium carbonate ______________________ 21. Maltose ______________________ 22. Magnesium hydroxide ___________________ 23. Sodium chloride _______________