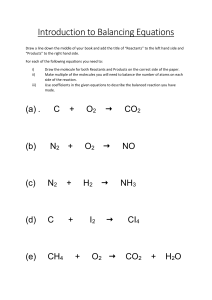
Balancing Chemical Equations In a chemical reaction reactants react to form products. Reactions are described by word equations and symbol equations. In a symbol equation the number of atoms of each element on the reactant side must be equal to the number of atoms of each element on the product side. (a balanced symbol equation) SO ........ the reaction between hydrogen gas and oxygen gas to make water can be written as: Word equation Hydrogen + Oxygen Water Unbalanced symbol equation H2 + O2 H2O Balanced symbol equation 2H2 + O2 2H2O (There are 2 atoms of hydrogen on each side but 2 of oxygen on the left and only 1 on the right. Putting a 2 in front of hydrogen and water resolves this.) (Note: you can only balance equations by putting a number in front of a formula NOT by changing the formula of a substance.) AND ........ the reaction between sodium metal and water to produce sodium hydroxide solution and hydrogen gas. Word equation Sodium + Water Sodium hydroxide + Hydrogen Unbalanced symbol equation Na + H2O NaOH + H2 (There is 1 atom of sodium and 1 atom of oxygen on each side but 2 of hydrogen on the left and 3 on the right. Putting a 2 in front of sodium, water and sodium hydroxide resolves this.) Balanced symbol equation 2Na + 2H2O 2NaOH + H2 (Note: you can only balance equations by putting a number in front of a formula NOT by changing the formula of a substance.) Balance these equations by putting the numbers of moles in the relevant boxes. (The first 2 have been done as examples.) 2 CuCO3 K CH4 Na Mg + + + + + Na2CO3 Fe C2H5OH Al CuO Al + + + + + + 2 2 HNO3 H2O O2 Cl2 HCl CaCO3 HCl O2 O2 Cl2 CH4 H2SO4 2 Cu(NO3)2 NaOH CO2 NaCl MgCl2 CaO NaCl Fe2O3 CO2 AlCl3 Cu Al2(SO4)3 + + + H2O H2 H2O + + + H2 CO2 CO2 + H2O + + CO2 H2 + CO2 + H2O + H2O Write balanced equations from the following word equations. (You’ll need to work out the formula for some of the substances or look them up on the sheet you’ve been given.) Potassium + Oxygen Potassium oxide Sodium hydroxide + Sulfuric acid Sodium sulfate + water Copper oxide + Nitric acid Copper nitrate + water