Properties of Water
advertisement

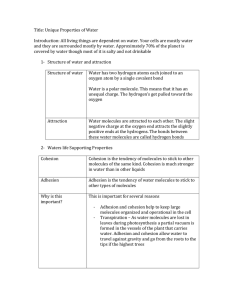
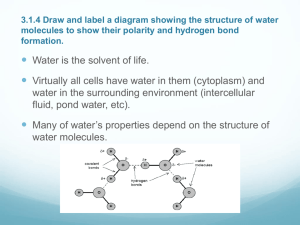
Learning Goals 1. You will be able to describe the structure of water. 2. You will be able to identify the 6 properties of water. 3. You will be able to describe how these properties are essential to sustaining life 2 Water 3 Types of Bonds: Hydrogen Bonds Water (H2O or H–O–H) is a polar molecule Electrons spend more time with O than H’s H’s become slightly +, O slightly – 4 Hydrogen Bonding Hold water molecules together The hydrogen bonds joining water molecules are weak, about 1/20th as strong as covalent bonds. They form, break, and reform with great frequency Extraordinary Properties that are a result of hydrogen bonds. Cohesive behavior Resists changes in temperature High heat of vaporization Expands when it freezes Versatile solvent 5 Think-pair-share Think about the following… What are the atoms that make up a water molecule? Which type of bonds hold the atoms together? What does polarity mean? Turn to your shoulder partner and share your answers 6 Properties of Water 1. 2. 3. 4. 5. 6. Polar Molecule Cohesion High Specific Heat Density- greatest at 4C Universal Solvent of Life Neutral pH 7 1. Polarity Water has a variety of unusual properties because of attractions between these polar molecules. The slightly negative regions of one molecule are attracted to the slightly positive regions of nearby molecules, forming a hydrogen bond. Each water molecule can form hydrogen This leads to Hydrogen 8 2. Cohesion When water molecules stick to other water molecules holding them together Why is this important? Organisms Depend on Cohesion Cohesion among water molecules plays a key role in the transport of water against gravity in plants Adhesion, clinging of one substance to another, contributes too, as water adheres to the wall of the vessels. 9 Surface Tension Surface tension, a measure of the force necessary to stretch or break the surface of a liquid, is related to cohesion. Water has a greater surface tension than most other liquids because hydrogen bonds among surface water molecules resist stretching or breaking the surface. 10 Surface Tension Some animals can stand, walk, or run on water without breaking the surface. 11 Water as a Transport Medium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Water evaporates, pulling the water column from the roots to the leaves. H2O Water molecules cling together and adhere to sides of vessels in stems. Water enters a plant at root cells. H2O 12 3. High Specific Heat • • • Moderate Temperatures on Earth Temperature is the measurement of the movement of molecules In order for molecules to move faster (get warmer) the hydrogen bonds holding the molecules together have to break. In order for the molecules to move slower (get cooler) the hydrogen bonds holding the molecules together have to form. 13 Specific Heat Specific Heat is the amount of heat that must be absorbed or lost for one gram of a substance to change its temperature by 1oC. Water has a High Specific Heat which means it takes a lot of energy to break or form the hydrogen bonds between water molecules 14 Specific Heat Why is this important? 1. Prevention of temperature changes that are outside the range suitable for life. 2. Coastal 3. A areas having a mild climate stable marine environment 15 Evaporative Cooling The cooling of a surface occurs when the liquid evaporates This is responsible for: Moderating earth’s climate Stabilizes temperature in aquatic ecosystems Preventing organisms from overheating 16 Ms. Laslow’s Random Fact of the Day!! Most animals cannot sweat like humans can. They have developed many ways to cope with the heat. Many of which involve evaporative cooling 17 Evaporative Cooling of Animals Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Calories of Heat Energy / g 800 Gas 600 540 calories 400 200 Liquid 80 calories Solid 0 freezing occurs 0 evaporation occurs 20 40 60 80 100 120 Temperature (°C) a. Calories lost when 1 g of liquid water freezes and calories required when 1 g of liquid water evaporates. b. Bodies of organisms cool when their heat is used to evaporate water. © Grant Taylor/Getty Images 18 4. Density More dense at 4C Contracts until 4C Expands from 4C to 0C Why is this important? 1. Prevents water from freezing from the bottom up. 2. Ice forms on the surface first—the freezing of the water releases heat to the water below creating insulation. 3. Makes transition between season less abrupt. 19 Density-Ice Frozen water less dense than liquid water Otherwise, oceans and deep lakes would fill with ice from the bottom up Ice acts as an insulator on top of a frozen body of water Melting ice draws heat from the environment 20 Density 21 A Pond in Winter Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ice layer Protists provide food for fish. River otters visit ice-covered ponds. Aquatic insects survive in air pockets. Freshwater fish take oxygen from water. Common frogs and pond turtles hibernate. 22 5. Universal Solvent Solutions consist of: A solvent (the most abundant part) and A solute (less abundant part) that is dissolved in the solvent Polar compounds readily dissolve; hydrophilic Nonpolar compounds dissolve only slightly; hydrophobic Ionic compounds dissociate in water Na+ Attracted to negative (O) end of H2O Each Na+ completely surrounded by H2O Cl Attracted to positive (H2) end of H2O Each Cl- completely surrounded by H2O 23 Universal Solvent Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. H O O H d - An ionic salt dissolves in water. Na+ H d O H d- d- O H H d+ d+ H O H H H d+ Cl– d+ H H O Science 360 24 Universal Solvent Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. H O H + d A polar molecule dissolves in water. - d + N + d H H d H H O H + d - d - d - d O H H O H H 25 6. Neutral pH pH scale used to indicate acidity and alkalinity of a solution. Values range from 0-14 0 to <7 = Acidic 7 = Neutral >7 to 14 = Basic (or alkaline) 26 The pH Scale Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. basic acidic H+ Ion Concentration 10 0 10–1 10–2 10–3 10–4 10–5 10–6 10–7 10–8 10–9 10–10 10–11 10–12 10–13 10–14 pH value 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Examples hydrochloric acid stomach acid, lemon juice vinegar, cola, beer tomatoes black coffee urine pure water seawater baking soda Great Salt Lake household ammonia household bleach sodium hydroxide 27 Buffers in Biology Health of organisms requires maintaining pH of body fluids within narrow limits Human blood normally 7.4 (slightly alkaline) Many foods and metabolic processes add or subtract H+ or OH- ions Reducing blood pH to 7.0 results in acidosis Increasing blood pH to 7.8 results in alkalosis Both life threatening situations Bicarbonate ion (-HCO3) in blood buffers pH to 7.4 28