Honors Formula Writing/Naming Chapters 7 and 9
advertisement

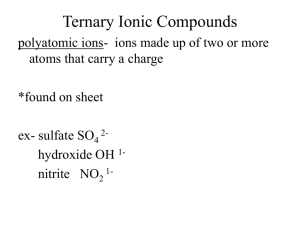
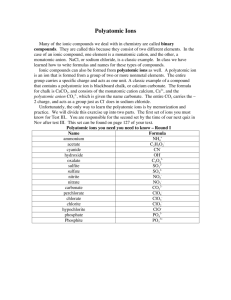
Honors Chapter 9 (and some Chapter 7) Chemical Names and Formulas Chemical formula - combination of symbols that represent the composition of a compound • Shows elements present and number of atoms subscripts • Represent the number of atoms of that element in the compound • No subscript is an “understood” 1 • • • • • • NaCl 1 Na 1 Cl H2SO4 2H 1S 4O Ca(ClO3)2 ????? 1 Ca 2 Cl 6O Ionic – transfer of electrons Called a “formula unit” Covalent – share electrons called “molecule” IONIC COMPOUNDS Four different types we will learn about Metal + nonmetal (binary ionic) Metal + polyatomic ion Polyatomic ion + polyatomic ion Polyatomic ion + nonmetal Monatomic ions – ions consisting of only one atom Charges can often be determined by using the periodic table Metallic elements – tend to lose electrons to form cations Group 1 all 1+ Group 2 all 2+ Nonmetals tend to gain electrons when they bond with metals – form anions Many have more than one common ionic charge Are going to use roman numerals Oxidation number • Indication of how many electrons it will gain or lose when it forms a bond • Gains or loses electrons – forms an ion • Charged particle • Can be found for each element on the periodic table • Refer to yours!!! • Some elements have more than 1 oxidation number – that means they can form more than one type of compound When a single atom takes on a charge (by gaining or losing electrons) – it forms a “monatomic ion” Ion made up of more than 1 atom – “polyatomic” ion Monatomic ions Na+ Ca+2 Cl- O-2 Polyatomic ions CO3-2 ClO3- OH- Why would an atom want to form an ion? Remember the “octet rule” Wants a filled outer shell For most atoms, that is 8 Samples on board using electron dot notation Na and Cl Ca and Cl Al and Cl Ca and S K and N you do 1) Metal + nonmetal Metal always written first – has positive oxidation number (written first) Nonmetal written second – has negative oxidation number Can use “criss-cross” method to arrive at correct formula. Must remember to simplify subscripts if possible!! Magnesium oxide - MgO Naming binary ionic compounds Metal full name first Nonmetal name with “ide” ending NaCl sodium chloride CaCl2 calcium chloride LiF lithium fluoride AlBr3 aluminum bromide Some metals have multiple oxidation numbers Use Roman numerals to specify the oxidation number used I, II, III, IV, V, VI, VII, VIII Transition metals characteristically have multiple oxidation numbers ONLY USE ROMAN NUMERALS IF THE METAL HAS MORE THAN ONE OXIDATION NUMBER LISTED Nonmetals may have more than one oxidation number, you just use the first number listed - NEGATIVE Examples: Co, Ni, Cu, Fe, Mn Always check before writing name for the compound FeCl2 Iron(II) chloride FeO Fe2O3 Write formulas for the following calcium sulfide strontium bromide chromium(III) chloride iron(II) oxide CaS SrBr2 CrCl3 FeO Name the following compounds Fe2O3 KI CuO NiCl3 CrO3 Iron(III) oxide Potassium iodide Copper(II) oxide Nickel(III) chloride Chromium(VI) oxide 2. Metal + polyatomic ion Almost all polyatomic ions have a negative charge 2 you are responsible for have a positive charge NH4+ and H3O+ Polyatomic ions travel as a unit Page 257 list of polyatomic ions NEVER CHANGE THE SUBSCRIPTS IN A POLYATOMIC ION THAT MEANS NEVER!!!!!!! Can use same “criss-cross” method for determining the correct formula Same rules apply – must factor the subscripts if you can (only the oxidation numbers that are used – NOT THE SUBSCRIPTS OF THE POLYATOMIC ION!!!!!! At first, always put a parenthesis around the polyatomic ion Only time the parenthesis can be dropped is if a “1” criss-crosses down or if the subscript factors to a “1” Don’t forget to include a roman numeral in the name if the metal has multiple oxidation numbers!!!!!! Examples on board Naming Metal name first (only use roman numeral if the metal has more than one oxidation number!!!) Second is the name of the polyatomic ion – taken directly from the table!! Don’t make up your own name!!!!!!!! Characteristics of Ionic Compounds • • • • • Representative unit = “formula unit” Type of elements: Metal with nonmetal Physical state: crystalline solid (hard) High melting point Most are soluble in H2O • Poor conductors of electricity in the solid state –But good conductors when melted (molten) or dissolved in H2O (aq) (ions free to move) Molecular Compounds (covalent) • Representative unit – “molecule” (bonded covalently) • Type of elements – nonmetals • Physical state – solid, liquid, gas – Solids – low melting point, brittle • Molecule – electrically neutral group of atoms that act as a unit Naming binary molecular compounds • 2 elements in the compound – Both nonmetals! • 2 naming systems – Prefix system – Stock system (roman numerals) • Both systems are correct • I’m sure you will prefer the prefix system! Prefixes you must memorize! Number of atoms 1 2 3 4 5 6 7 8 9 10 Prefix used mono di tri tetra penta hexa hepta octa nona deca • When 2 nonmetallic elements combine – Often do so in more than one way – Example CO CO2 • Problem with calling them both “carbon oxide” • CO2 – you exhale. It is normally present in the air you breathe • CO – hopefully is not in the air you breathe • In large amount R.I.P. • Catalytic converter – cars – Converts CO to CO2 Naming binary molecular compounds • Prefix + first element name • Followed by prefix + 2nd element name with “ide” ending • ******only time you can neglect a prefix is if the first element in the compound is a single atom • PCl3 phosphorus trichloride • CO • Carbon monoxide (not monocarbon monoxide!) • • • • Don’t use “double vowels” Change if a “tongue twister” Monooxide monoxide Decaoxide decoxide • Trioxide – is fine • • • • • • N2O Dinitrogen monoxide SF6 Sulfur hexafluoride N2H4 Dinitrogen tetrahydride – NO FACTORING ALLOWED!!! • P2O3 • Diphosphorus trioxide Name the following: • • • • • CS2 Cl2O7 N2O5 CCl4 CrCl3 • • • • • Carbon disulfide Dichlorine heptoxide Dinitrogen pentoxide Carbon tetrachloride Chromium III chloride GOTCHA!!!!! Write formulas for the following: • • • • carbon tetrabromide dinitrogen tetrahydride boron trichloride diphosphorus trioxide • • • • CBr4 N2H4 BCl3 P2O3 • A molecular compounds worksheet just for you!! Network covalent compounds • Covalent compounds that are not made up of individual molecules. • All the atoms are joined in a covalently bonded three dimensional network • No distinct units in these compounds • Have very high melting points • SiC SiO2 Si3N4 Acids you should know! • Oxyacids – contain H, O and a third element (usually a nonmetal) • Acetic HCH3COO (acetate ion) • Nitric HNO3 (nitrate ion) • Nitrous HNO2 (nitrite ion) • Phosphoric H3PO4 (phosphate ion) • • • • • • • Sulfuric Sulfurous Carbonic Hypochlorous Chlorous Chloric Perchloric H2SO4 H2SO3 H2CO3 HClO HClO2 HClO3 HClO4 (sulfate ion) (sulfite ion) (carbonate ion) (hypochlorite ion) (chlorite ion) (chlorate ion) (perchlorate ion) HF HCl HBr HI hydrofluoric acid hydrochloric acid hydrobromic acid hydroiodic acid