Origins of Life
advertisement

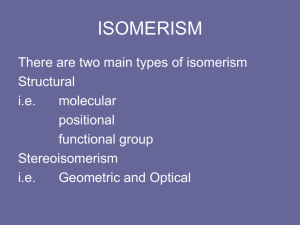
1 ORGANIC CHEMISTRY 2 Types of Organic Compounds • Vast majority of over 20 million known compounds are based on C: organic compounds. • Generally contain C and H + other elements • Great variety of compounds Isomerism • Isomers have identical composition but different structures • Two forms of isomerism – Constitutional (or structural) – Stereoisomerism • Constitutional – Same empirical formula but different atomto-atom connections • Stereoisomerism – Same atom-to-atom connections but different arrangement in space. 3 4 Structural Isomers 5 Stereoisomers: Geometric Geometric isomers can occur when there is a C=C double bond. Cis-2-butene Trans-2-butene 6 Stereoisomers: Optical • Optical isomers are molecules with non-superimposable mirror images. • Such molecules are called CHIRAL • Pairs of chiral molecules are enantiomers. • Chiral molecules in solution can rotate the plane of plane polarized light. Chiral Compounds and Polarized Light 7 8 Stereoisomers Isomers Chirality generally occurs when a C atom has 4 different groups attached. Lactic acid Stereoisomers Isomers Lactic acid isomers are nonsuperimposable 9 Chirality: Handedness in Nature These molecules are non-superimposable mirror images. 10 Chirality: Handedness in Nature These amino acids are nonsuperimposable mirror images. 11 Stereoisomers in Nature Right- and lefthanded seashells The DNA here is right-handed 12 13 Hydrocarbons • Compounds of C and H • Subgroups: – Alkanes: C-C single bonds – Alkenes: C=C double bonds – Alkynes: carbon-carbon triple bonds – Aromatic: based on benzene 14 Hydrocarbons • Alkanes have the general formula CnH2n+2 • CH4 = methane • C2H6 = ethane • C3H8 = propane • C4H10 = butane • C5H12 = pentane 15 16 Methane Hydrate, CH4(H2O)x Methane Hydrate, CH4(H2O)x 17 18 Methane Hydrate, CH4(H2O)x Gas hydrates have been known for many years, and combustion of a sample of methane hydrate is seen on the front cover. Recently, however, vast deposits of methane hydrate were discovered deep within sediments on the floor of the world’s oceans. How these deposits were formed is a mystery. But what is important is their size. It is estimated that the global methane hydrate deposits contain approximately 1013 tons of carbon, or about twice the combined amount in all reserves of coal, oil, and conventional natural gas. Now if scientists and engineers could only solve the problem of extracting the methane conveniently and safely! CH3CH2 CH2CH2 CH3 Pentane Hydrocarbons & Structural Isomerism 19 CH3 CH3CHCH2CH3 2-Methylbutane CH3 H3CCCH3 CH3 2,2-Dimethylpropane Note names of isomers Isomers of C5H12? C5H12 has 3 structural isomers. C6H14 has 5 C7H14 has 9 20 Hydrocarbons: Alkanes Alkanes are colorless gases, liquids, and solids Generally unreactive (but undergo combustion) Not polar (or low polarity) and so are not soluble in water. 21 Hydrocarbons: Cycloalkanes All compounds are flexible. Cyclohexane, C6H12, has interconverting “chair” and “boat” forms. Axial H ato m H H 4 H H Eq u ator ial H atom H 5 6 2 3 H H H H 1 HH Chair form 4 H H 1 H 6 5 H 3 H 2 H H H H H H H Boat form H H H H 5 4 H 3 HH H H 1 6 H H 2 H Chair form H 22 Alkenes: Compounds with C=C Double Bonds • How many isomers are possible for a compound with the formula C4H8? 3 4 CH2CH3 H 1 C H 1 C 2 C H H 1-butene 3 CH3 H 1 H3C 2 C 4 CH3 2 C CH3 2-methylpropene (isobutene) H 4 CH3 H 3 C 2 C H cis-2-butene 1 H3 C 3 C H trans-2-butene 23 Alkenes— Many Occur Naturally 24 Reactions of Alkenes: ADDITION REACTIONS • Alkenes are unsaturated — more bonds can form to the C atoms • Molecules such as Br2, H2, HCl, HBr, and H2O add to the double bond H C C + Br2 H H H Br Br H C C H H H 1,2-dibromoethane An Addition Reaction Fat placed in Br2 vapor • The fat in bacon is partially unsaturated. The fat adds Br2 to the C=C bonds. • Fats can be “hydrogenated” with H2. 25 An Addition Reaction Fat placed in Br2 vapor 26 An Addition Reaction • Fats can be “hydrogenated” with H2. Peanut butter has partially hydrogenated vegetable oil. 27 Alkynes • Alkynes have carbon-carbon triple bonds. • C2H2: common name = acetylene systematic name = ethyne Preparation: CaC2(s) + H2O(liq) --> C2H2 (g) + Ca(OH)2(s) ∆Hfo(C2H2, g) = +226.7 kJ/mol ∆Hrxn for C2H2 + O2 = –1300 kJ/mol 28 29 Aromatic Compounds • Benzene, C6H6, in the top 25 chemicals produced in the U.S. • Starting point for hundreds of other compounds. 30 Resonance in Benzene • C6H6 has two resonance structures with alternating double bonds. • The π electrons are delocalized over the ring. H C HC H C C C H H CH H H C C H C C H CH C H Resonance structures of benzene, C6 H6 H C HC H C C C H H CH Abbreviated representation of resonance structures Resonance in Benzene • CC bond order is _______________ • C–C single bond = 154 pm C=C bond = 134 pm • CC bonds in benzene = 139 pm π electrons delocalized 31 32 Other Aromatic Hydrocarbons Toluene Naphthalene Benzene Derivatives 33 Aniline Phenol C6H5NH2 TNT trinitrotoluene C6H5OH C6H4CH3(NO2)3 Naming Benzene Derivatives Cl 1 6 2 3 5 4 34 Ortho to Cl Meta to Cl Para to Cl 1,4-dimethylbenzene Common name: Para-xylene Reactions of Aromatics 35 • Substitutions — not additions — are typical. CH3 + CH3Cl AlCl3 AlCl3 is a catalyst. Catalysts typically used in aromatic substitutions. + HCl 36 Functional Groups See CD-ROM Screens 11.5 & 11.6 37 Alcohols • Characterized by –OH group • Name: add –ol to name of hydrocarbon Methanol Butanol 38 Structures of Alcohols C3H5OH: how many structural isomers? H H H H C C C H H H 1-propanol OH H H OH H C C C H H H H 2-propanol Naming: Add -ol to name of 3-C hydrocarbon. Indicate position of OH with number. 39 Alcohol Properties • Alcohols are a derivative of water • Many alcohols dissolve in water Methanol dissolves in water. Butanol is NOT soluble in water. 40 “Sterno” • Alcohols burn in air • A mixture of ethanol + calcium acetate = STERNO GLYCOLS Alcohols with Two OH Groups Ethylene glycol Propylene glycol 41 42 Alcohol Reactions Screen 11.6 Substitution Elimination—the reverse of addition 43 Sugars: Related to Alcohols • Sugars are carbohydrates, compounds with the formula Cx(H2O)y. CHO H OH 4 HO HO 5 3 H H OH 2 H 3 H OH 4 H OH 5 H HO 2 HO 1 OH OH a-D-glucose H 1 CH2OH H OH 4 HO HO 5 HO 1 3 H H 2 OH H b-D-glucose Open chain form What is the difference between a and b D-glucose? OH 44 Sucrose and Ribose H OH HO HO HO H OH O H HO H O H CH2OH a-D-Glucose H HO H O OH Fructose H OH H CH2 OH OH H H H Ribose, a pentose in the DNA backbone 45 Amines Alcohols are derivatives of H2O (R–OH) and amines are derivatives of NH3. Methylamine Dimethylamine Trimethylamine 46 Amines Amines generally have terrible odors! Cadaverine Pyridine Amines 47 Amines, like NH3, are bases 2 C6H5NH2 (aq) + H2SO4(aq) Aniline 2 C6H5NH3 +(aq) + SO42-(aq) Anilinium ion 48 Amines H+ Nicotine Many natural products and drugs (such as nicotine and cocaine) are bases. 49 O C Aldehyde Compounds with Carbonyl Group Carboxylic acid Ketone 50 Structures of Aldehydes Cinnamaldehyde Odors from aldehydes and ketones Carboxylic Acids Acetic acid Acids are found in many natural substances: bread, fruits, milk, wine 51 Benzoic acid Carboxylic acid group with acidic H+ All are WEAK acids 52 Carboxylic Acids H O Formic acid, HCO2H, gives the sting to ants. C O O C CH3 O Aspirin, acetylsalicylic acid 53 Acids + Alcohols --> ESTERS Esters have generally pleasant odors 54 Acids + Alcohols --> ESTERS O O CH 3COH + CH 3CH 2OH Acetic acid H+ CH 3COCH 2CH 3 + H 2O Ethyl acetate Ethanol O O RC—O—H + R'—O—H Carboxylic acid Alcohol H+ RC—O—R' + H 2O Ester One of the important reactions in nature! 55 Acids + Alcohols --> ESTERS 3-methylbutanol Acetic acid O H3C C CH3 O CH2 CH2CHCH3 3-methylbutylacetate Glycerol Alcohol with 3 OH Groups Combine this with long chain acids ------> ??? Fatty acids ---> fats and oils 56 Fats and Oils H2 C HC H2 C O O CR O O CR O O CR What is the functional group in a fat or oil? R = organic group with NO C=C bonds C12 = Lauric acid C16 = Palmitic acid C18 = Stearic acid R = organic group with C=C bonds C18 = oleic acid 57 H2 C HC H2 C O O CR O O CR O O CR Fats and Oils 58 Fats with C=C bonds are usually LIQUDS Oleic acid: a monounsaturated fatty acid C=C bond H2 C HC H2 C O O CR O O CR O O CR Fats and Oils Fats with saturated acids (no C=C bonds) are SOLIDS. Saturated fats are more common in animals. 59 Fats and Polar Bears 60 •Bears gorge on blubber in the winter. •During the summer bears rely on stored fat for energy. •Burn 1-1.5 kg of fat per day. •Water for metabolism comes from fat burning. Trans Fatty Acids 61 •Oleic acid is a mono–unsaturated cisfatty acid •Trans fatty acids have deleterious health effects. •Trans fatty acids raise plasma LDL cholesterol and lower HDL levels. C=C bond 62 Fats and Oils: Saponification Glyceryl stearate, a fat + NaOH O CH2 O CR O + 3 NaOH CH O CR O CH2 O CR R = —(CH2 )16CH3 CH2 OŃ H CH OŃ H CH2 OŃ H Glycerol O + 3 RCŃ O- Na+ Sodium stearate, a soap 63 Acids + Amines --> AMIDES N-methylacetamide 64 Acids + Amines --> AMIDES H C H H H O H O C C C C C C N C H H H H Amide link Acetoaminophen Tylenol, Datril, Momentum, ... 65 Alpha-Amino Acids H2N H O C C OH R Amine H Alanine H3C C Chiral a-carbon NH3 CO2 Acid 66 Peptides and Proteins O H 3N OŠ H CH3 Alanine HOCH 2 H 3N + H OŠ O Serine peptide bond H HOCH2 H H 3N O N H O OŠ CH3 Adding more peptide links ---> PROTEIN Polymers • Giant molecules made by joining many small molecules called monomers • Average production is 150 kg per person annually in the U.S. 67 68 Polymer Classifications • Thermoplastics (polyethylene) soften and flow when heated • Thermosetting plastics — soft initially but set to solid when heated. Cannot be resoftened. • Other classification: plastics, fibers, elastomers, coatings, adhesives 69 Polymer Preparation • Addition polymers — directly adding monomer units together • Condensation polymers — combining monomer units and splitting out a small water (water) 70 Polyethylene: Addition Polymer n H2 C CH2 Ethylene H H C C H H n Polyethylene A polymer with a molar mass of 1e6 has about 36,0000 units. Mechanism of Addition Polymerization 71 72 Types of Polyethylene Linear, high density PE (HDPE) Branched, low density PE, LDPE Cross-linked PE, CLPE 73 Types of Polyethylene Polymers based on Substituted Ethylenes, CH2=CHX CH2CH OH CH2CH n polyvinyl alcohol CH2CH OCCH3 n O polyvinyl acetate n polystyrene Table 11.12: others are PVC, acrylonitrile, polypropylene, polymethyl methacrylate Polystyrene • Polystyrene is nonpolar material and dissolves in organic solvents. • PS foam is mostly air, and when it dissolves it collapses to a much smaller volume. 74 75 Slime! Slime is polyvinylalcohol cross-linked with boric acid 76 Condensation Polymers O n HOC O COH + n HOCH2CH2OH terephthalic acid O C ethylene glycol O COCH2CH2O + H2 O n Polyethylene terephthalate (PET), a polyester 77 Polyesters, PET Jackets made from recycled PET soda bottles Soda bottles, mylar film. 78 Polyesters: Mechanism 79 Polyamides: Nylon 80 Polyamides: Nylon •Each monomer has 6 C atoms in its chain. •A polyamide link forms on elmination of HCl •Result = nylon 66 •Proteins are polyamides 81 Polymer Recycling Symbols LDPE = HDPE = PP = V= Low density PE = 0.910-0.925 g/cm3 High density PE = 0.941-0.965 Polypropylene = 0.90 PVC (Vinyl chloride) = 1.30-1.58