05._PhononsThermalProperties
advertisement

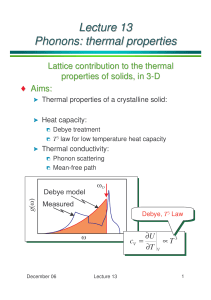
5. Phonons Thermal Properties • Phonon Heat Capacity • Anharmonic Crystal Interactions • Thermal Conductivity Phonon Heat Capacity • • • • • • • • Planck Distribution Normal Mode Enumeration Density of States in One Dimension Density of States in Three Dimensions Debye Model for Density of States Debye T3 Law Einstein Model of the Density of States General Result for D(ω) dQ dU PdV U dT U dV PdV T V V T U U V U V P dT P dP T V V T T P V T P T Q U CV T V T V Q V U U CP P T T P T V V T P S V CV T V T T P 2 P V P V CV T CV T T V T P V T T P where P 1 V V T P P2 CV TV T = Thermal expansivity 1 V T = isothermal compressibility = 1/ B V P T CP CV BTV P2 9BTV 2 B = Bulk modulus α = linear (1-D) thermal expansivity Lattice heat capacity: U lat Clat T V U lat U K , p nK , p K p K Planck distribution: n p 1 e / k BT 1 K , p p = polarization Planck Distribution P E e E / kB T System at constant T Canonical ensemble : Boltzmann factor N n 1 E E /k T e n1 n B e Nn For a set of identical harmonic oscillators / kB T Nn = number of oscillators in the nth excited state when system is in thermal equilibrium Pn Probability of an oscillator in the nth excited state: Nn N s 0 Occupation number: n nPn n 0 ne n0 e n / kB T s / kB T s 0 1 x 1 x s 0 s x e / kB T x d s sx x x 2 dx 1 x s 0 s 0 s n x 1 x e 1 / k BT 1 s e n e s 0 / kB T s / kB T Normal Mode Enumeration U lat K p K , p e K , p / k BT Clat d Dp p 1 d D p p e /k T k BT 2 e 2 B / k BT 2 1 2 e / k BT k B d D p 2 k T p B e / kBT 1 e / k BT 1 Density of States in One Dimension Fixed boundary problem of N+1 particles. N = 10 L Na u0 t uN t 0 → u K , p, t u p, 0 eiK , p t sin sKa s u p, 0 e i K , p t sin with K m sm N L m Na m 0,1,2, , N Number of allowed K for non-stationary solutions is N–1 = Number of mobile atoms Polarization p : 1 long, 2 trans DK L /a / Na dK D K N 1 Periodic boundary problem of N particles L Na N=8 us t uN s t → with u s K , p , t u p, 0 e K 2m L i K , p t i s K a 2m Na us K , p, t u p, 0 e e K a i K , p t i s 2 m / N e → m 0, 1, , N 1 N , 2 2 K are the same a Number of allowed K for non-stationary solutions is N = Number of mobile atoms L DK 2 /a /a dK D K N fixed B.C. /a max 0 0 D K dK DK dK DK d d L → max D d 0 D D K dK L 1 d vG Periodic B.C. max /a dK D K dK D K d d / a max DK L 2 → max D d 0 D 2 D K dK L 1 d vG Density of States in Three Dimensions Periodic B.C. ; N 3 cells in cube of side L → density of states in K-space is 3 V L D K 3 2 8 Number of modes per polarization lying between ω and ω + d ω is D d d K D K 3 K d → density of states in ω-space is For isotropic materials, D V 3 8 dK d 2 S K V d 2S 3 d 8 K K V d 2S D 3 8 K K K K V 2 dK 4 K 8 3 d → K V d 2S 3 8 K vg d dK Debye Model for Density of States Debye model: K vK v velocity of sound (for a given type of polarization) V K2 2 2 v V dK D 3 4 K 2 8 d V 2 2 3 2 v For a crystal of N primitive cells: N D D d 0 D V 2 v 2 3 d 0 2 V D3 2 3 6 v 1/3 → N D 6 2 v 3 V D Debye frequency 1/3 N KD 6 2 v V Thermal (vibrational) energy U D d D n 0 U where D V 2 v 2 3 x d 2 e 0 / kBT V kB4T 4 2 3 3 1 2 v xD k BT D k BT θ Debye temperature T U 3Nk BT V CV 2 3 2 v D 0 3 xD x3 0 dx e x 1 dx 0 Debye integrals: See Ex on Zeta functions, Arfken T D kB x3 0 dx ex 1 1/3 v 6 2 N kB V 3 xD 3 d T e T 3NkB xD / k BT V 2 2 v3 1 x 4e x e x 1 for each acoustic branch 2 D 0 d e 3 / k BT 1 k BT 2 2 e / k BT Debye model 1 cal/mol-K 4.186 J/mol-K Debye T3 Law For low T, xD → : → T U 3Nk BT x3 4 4 3 s x 6 s 6 4 e 0 dx e x 1 0 dx x 15 s 1 s 1 3 xD x3 0 dx e x 1 4 4 T CV Nk B 5 3 4 T Nk BT 5 T 78 Nk B so that for each acoustic branch 3 To account for all 3 acoustic branches, we set and 3 1/3 v 6 2 N kB V V 2 D 2 3 vp p 2 1 1 2 kB V 3 3 2 3 3 vL vT 6 N 3 1 12 4 T CV Nk B 5 3 T 234 Nk B 3 Good for T < θ /50 Solid Ar, θ = 92K c.f. Table for Bulk Moduli, Chap 3, p.52 Qualitative Explanation of the T3 Law Of the 3N modes, only a fraction (KT /KD ) 3 = ( T / θ )3 is excited. U T 3 Nk BT 3 → T C 12 Nk B 3 Einstein Model of the Density of States N oscillators of freq ω0. U D d D n D N 0 N n 0 0 0 N 0 e 0 / k B T 1 2 0 U e 0 / kBT CV Nk B T k T V B e 0 / kBT 1 2 Diamond E 0 kB 1320 K Classical statistical mechanics: Dulong-Petit value CV = 3NkB General Result for D(ω) V d 2S D 3 8 K vg Si Debye solid vg ~ 0 Van Hove Singularities Anharmonic Crystal Interactions Harmonic (Linear) Waves: • Normal modes do not decay. • Normal modes do not interact. • No thermal expansion. • Adiabatic & isothermal elastic constants are equal. • Elastic constants are independent of P and T. • C → constant for T > θ . Deviation from harmonic behavior → Anharmonic effects Thermal Expansion U cx 2 gx3 fx 4 1-D anharmonic potential: x High T: dx x e dx e U Boltzmann distribution U 1 k BT g, f dx x n e a x 1 2 4 dx x e a x 2 g x e c x 1 g x3 f x4 e U → c 2 a n 1 2 n 1 i n 1 e 2 3 4 5 c c even n 1!! n n 1 for n 2 a odd 0 3 4 a5 dx e 3g 3g kT 4 c 2 4c 2 B a x2 a Thermal Expansion c=1 g = .2 f = .05 Lattice constant of solid argon Thermal Conductivity Heat current density: J Q T κ = Thermal conductivity coefficient For phonons, JQ = JU . Key features of kinetic theory (see L.E.Reichl, “A Modern Course of Statistical Mechanics”, §13.4 ): 1.Quantities not conserved in particle collisions are quickly thermalized to (global) equilibrium values. (e.g., velocity directions & magnitudes ) 2.Conserved quantities can remain out of global equilibrium (e.g., stay in local equilibrium. They get transported spatially in the presence of a “gradient”. 3.MFP is determined by collisions that do not conserve the total momenta of particles. Net amount of A(z) transported across the x-y plane at z0 in the +z direction per unit area per unit time: n v A z0 z A z0 z 2n v z dA dz 2n v al dA dz Δz = distance above/below plane at which particle suffered last collision. n = particle density, l = mean free path, a = some constant. J A z bA n v l dA dz 1 J A n v l A 3 bA = 1/3 is determined from self diffusion v v For heat conduction, we set 1 dT JQ z n v l c 3 dz 1 3 1 3 nvlc C vl AcT where c = heat capacity per particle v v = sound velocity C = n c = heat capacity Thermal Resistivity of Phonon Gas Harmonic phonons: mfp l determined by collisions with boundaries & imperfections. Anharmonic phonons: only U-processes contribute. Gas: Elastic collisions. No T required. κ= Gas: No net mass flow. Inelastic collisions with walls sets up T & n gradients. Finite κ. Crystal: N-processes only. κ=. Crystal: U-processes. Finite κ. Umklapp Processes 2-D square lattice Normal processes: K1 K 2 K 3 Energy is conserved: Umklapp processes: K1 K 2 K 3 G 1 2 3 Condition: G0 1 K1 , K 2 G 2 T > θ: all modes excited → no distinction between N- & U- processes → l 1/T. T < θ: probability of U- processes & hence l 1 exp(–θ /2T). Imperfections Low T → umklapp processes negligible. Geometric effect dominates. Size effect: l > D = smallest dimension of specimen. K C v D T3 Dielectric crystals can have thermal conductivities comparable to those of metals. Sapphire (Al2O3): κ ~ 200W cm–1 K–1 at 30K. Cu: max κ ~ 100W cm–1 K–1. Metalic Ga: κ ~ 845W cm–1 K–1 at 1.8K. ( Electronic contributions dominate in metals. ) Highly purified c-NaF Isotope effect on Ge. Enriched: 96% Ge74. Normal: 20% Ge70 , 8% Ge73 , 37% Ge74 , 8% Ge76.