Relative Reactivities, Structures and Spectra of Carboxylic Acid
advertisement
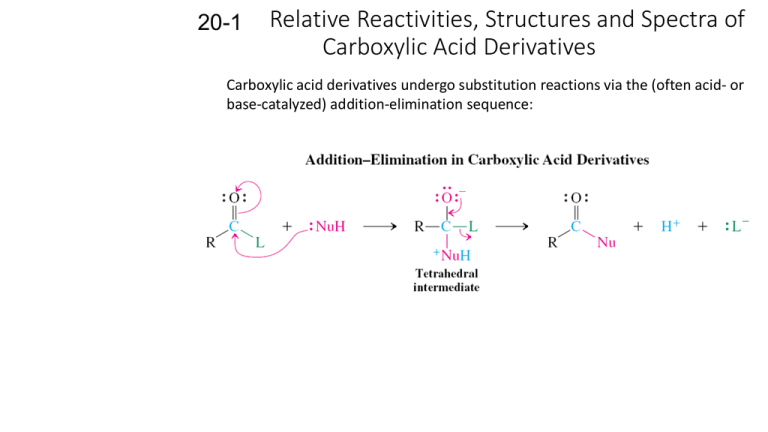
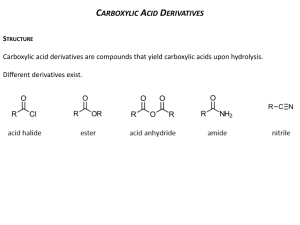
20-1 Relative Reactivities, Structures and Spectra of Carboxylic Acid Derivatives Carboxylic acid derivatives undergo substitution reactions via the (often acid- or base-catalyzed) addition-elimination sequence: The relative reactivities of the substrates follow a consistent order: The order of reactivity depends upon the ability of L to act as a leaving group and what effect it has on the adjacent carbonyl function. Lone pairs on L can be delocalized onto the carbonyl oxygen: The resonance form on the right is most important in amides and somewhat less important in esters. Amides and esters are strongly stabilized by resonance. Anhydrides are more reactive than esters because the lone pairs on the central oxygen are shared over two carbonyl groups. Alkanoyl halides are least stable because of their electronegatives and the poor overlap between their p-orbitals and those of carbon. The NMR spectra of N,N-dimethylformamide at room temperature exhibits two singles for the two methyl groups. •Bond rotation about the C-N bond in this molecule is very slow on the NMR time scale. •The measured barrier to this rotation is about 21 kcal mol-1. The amide nitrogen possesses sp2 hybridization. The resultant planarity of the amide group is the most important determinator of structure (thus, function) in peptides and proteins. IR spectra of amides and esters also indicate the presence of resonance in the structures. The C=O bond is weakened, which causes a corresponding decrease in the carbonyl stretching frequency. The IR spectra of monomeric acetic acid displays a carbonyl stretching frequency of 1780 cm-1, similar to that of anhydrides. The 13C NMR signals of the carbonyl carbons in carboxylic acid derivatives are less sensitive and fall into a narrow range near 170 ppm. Carboxylic acid derivatives are basic and acidic. Resonance in carboxylic acid derivatives affects their basicity (protonation at the carbonyl oxygen) and their acidity (enolate formation). Protonation becomes easier as L becomes more electron-donating. 20-2 Chemistry of Alkanoyl Halides The alkanoyl halides are named after the alkanoic acid from which they are derived. The halides of cycloalkanecarboxylic acids are called cycloalkanecarbonyl halides. Alkanoyl halides undergo addition-elimination reactions: Water hydrolyzes alkanoyl chlorides to carboxylic acids. Alkanoyl chlorides react with water to give the corresponding carboxylic acids and hydrogen chloride. Alcohols convert alkanoyl chlorides into esters. Esters can be effectively produced by the reaction of alkanoyl chlorides with alcohols. An alkali metal hydroxide, pyridine or a tertiary amine is usually added to neutralize the HCl produced by the reaction. The basic or neutral conditions employed in this method avoid the equilibrium problem of acid-catalyzed ester formation. Amines convert alkanoyl chlorides into amides. Ammonia, primary amines and secondary amines convert alkanoyl chlorides into amides. Aqueous ammonia can be used for the synthesis of simple amines since it is a much stronger nucleophile than water. The HCl formed is neutralized by a base, which can be excess amine. The mechanism of amide formation from alkanoyl chlorides is additionelimination: Tertiary amines cannot form amides since they do not possess a proton to lose during the last step of the reaction.