Williamson Ether Synthesis
advertisement

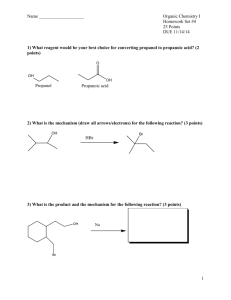
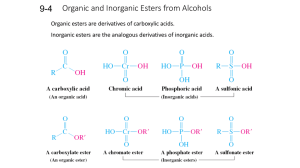
9-6 Williamson Ether Synthesis Ethers are prepared by SN2 reactions. Ethers can be prepared by the reaction of an alkoxide with a primary haloalkane or sulfonate ester under SN2 conditions. The parent alcohol of the alkoxide can be used as the solvent, however other polar solvents are often better, such as DMSO (dimethyl sulfoxide) or HMPA (hexamethylphosphoric triamide). The use of alkoxides in ether synthesis is restricted to primary unhindered alkylating agents, otherwise significant amounts of E2 products are formed. Cyclic ethers can be prepared by intramolecular Williamson synthesis. Haloalcohols serve as the starting point for the Williamson synthesis of cyclic ethers. The intramolecular reaction is usually much faster than the intermolecular reaction. If necessary, the intermolecular reaction can be suppressed by using a high dilution of the haloalcohol. Cyclic ethers of even small rings can be prepared using the Williamson synthesis. Ring size controls the speed of cyclic ether formation. The rate of ring closure is based on both enthalpic and entropic contributions. These rate differences can be explained based on the interplay between strain, entropy, and proximity. Entropy reduction (due to ring closure) increases with increasing ring size. (Reaction rate decrease with increasing ring size.) Ring strain decreases with increasing ring size. (Reaction rate increase with increasing ring size.) Transition-state strain is reduced in the 2-haloalkoxides because the 2-haloalkoxide is already strained by the proximity of the halide and hydroxyl. (Reaction rate increase for the 2-haloalkoxides.) The intramolecular Williamson synthesis is stereospecific. Since the Williamson synthesis is a SN2 substitution reaction, an inversion of configuration occurs at the carbon bearing the leaving group. The leaving group must be on the opposite side of the molecule from the attacking nucleophile in order for the reaction to occur. 9-7 Synthesis of Ethers: Alcohols and Mineral Acid Alcohols give ethers by both SN2 and SN1 mechanisms. Strong nucleophilic acids (HBr, HI) yield haloalkanes when reacted with alcohols. Strong non-nucleophilic acids yields ethers when reacted with alcohols. At higher temperatures, an E2 elimination of water occurs with the subsequent production of alkenes. Secondary and tertiary alcohols form ethers through an SN1 reaction with a second molecule of the alcohol trapping the carbocation. The E1 pathway becomes dominate at higher temperatures. Mixed ethers containing one tertiary and one primary or secondary alcohol can be prepared in the presence of dilute acid. The tertiary carbocation is trapped by the less hindered alcohol. Ethers also form by alcoholysis. This occurs by simply dissolving a tertiary or secondary haloalkane in an alcohol and waiting until the SN1 process is complete. 9-8 Reactions of Ethers Ethers are usually inert, however, they do react slowly with oxygen to form hydroperoxides and peroxides which can decompose explosively. 9-8 Reactions of Ethers The ether oxygen atom can be protonated to generate alkyloxonium ions. With primary groups and strong nucleophilic acids (HBr), SN2 displacement takes place. Oxonium ions from secondary ethers may transform by either SN2 or SN1 reactions, depending upon conditions. Tertiary ethers are protecting groups for alcohols. Esters containing tertiary alkyl groups react in dilute acid to give carbocations which are either trapped (SN1) by good nucleophiles or deprotonated in the absence of good nucleophiles. Because they are readily formed (and equally readily hydrolyzed), tertiary ethers are commonly used as protecting groups during chemical reactions which might otherwise interact with the unprotected alcohol.