Matter and Energy
advertisement

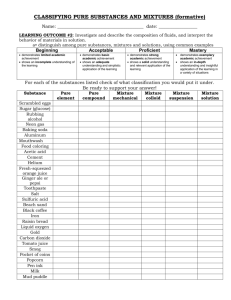
Why Does Matter Matter? TEKS • 4 (A) differentiate between physical and chemical changes and properties; • 4 (B) identify extensive and intensive properties; • 4 (D) classify matter as pure substances or mixtures through investigation of their properties. Physical vs. Chemical Changes • Examples: – rusting iron • Chemical – dissolving in water • Physical – burning a log • Chemical – melting ice – grinding spices • Physical • Physical Sure Signs of a Chemical Change • Energy Change (Temperature and Light) • Gas Produced (not from boiling!) • Precipitate – a solid formed by mixing two liquids together • Color Change (Be Careful) http://jchemed.chem.wisc.edu/JCESo ft/CCA/CCA0/MOVIES/S1047.MOV Physical Changes – can be observed without changing the identity of the substance Some physical changes would be • Phase Change – boiling of a liquid – melting of a solid • dissolving a solid in a liquid to give a homogeneous mixture — a SOLUTION. Changes Quiz Are the following changes considered chemical or physical? • 1. Sharpening a pencil • 2. Dissolving Koolaid in water • 3. Silver Tarnishing • 4. Alcohol evaporating • 5. Milk souring Chemical Properties and Chemical Change •Burning hydrogen (H2) in oxygen (O2) gives H2O. • Chemical change or chemical reaction — transformation of one or more atoms or molecules into one or more different molecules. Physical Properties ( see STAAR field guide) What are some physical properties? • color • melting and boiling point • odor Graphite — layer structure of carbon atoms reflects physical properties. Physical vs. Chemical Properties • Examples: – melting point physical – flammable chemical – density physical – magnetic physical – tarnishes in air chemical Physical Chemical Properties quiz 1. 2. 3. 4. 5. 6. 7. 8. Combustible Hard 13 Kilograms Boils at 200°C Density of 6.1g/cm³ Corrodes in air Soluble in water Volatile in water Chemical Physical Physical Physical Physical Chemical Physical Chemical What are Extensive and Intensive Properties? All Physical Properties of Matter can be classified as Extensive or Intensive. • Extensive Properties- depend on the amount of matter Ex) Mass • Intensive Properties- DOES NOT depend on the amount of matter. Ex) luster, density (Mass/Volume ratio) Determine Extensive or Intensive from the Physical properties on slide 9. Intensive/Extensive Properties Quiz • • 1. 2. 3. 4. Hard 13 Kilograms Boils at 200°C Density of 6.1g/cm³ Corrodes in air Soluble in water Mixtures and Pure Substances • Matter can be classified as a mixture or a pure substance. Mixtures • A mixture has variable composition • • A homogeneous mixture has the same properties throughout. • A heterogeneous mixture has different properties in different parts of the mixture. Separation of Mixtures distillation filtration Separation of Mixtures Decantation- is a process of carefully pouring a solution from a container in order to leave the precipitate (sediments) in the bottom of the original container. Chromatography- involves passing a mixture dissolved in a "mobile phase" through a stationary phase. Chromatography works on the principle that different compounds will have different solubilities and adsorption to the two phases, which will allow for their separation. Pure Substances A pure substance always has the same composition. • Pure substances are of two types: Elements which cannot be broken down chemically into simpler substances Compounds which can be chemically broken down into elements Water is a compound. All the components are the same—H2O molecules. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) no Can it be physically separated? PURE SUBSTANCE no Heterogeneous Mixture yes Can it be chemically decomposed? Compound no Element How can it be separated ? How can it be separated ? Filtration Chemically Distillation Electrolysis Chromatography Nuclear Reaction Types of Mixtures • Variable combination of 2 or more pure substances. Heterogeneous – visibly separate phases Homogeneous – Same throughout