File
advertisement

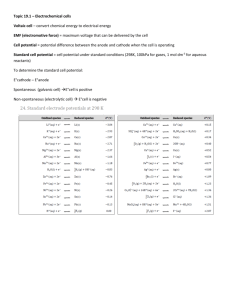
Corrosion • • • • • Corrosion of Iron Since Ered(Fe2+) < Ered(O2) iron can be oxidized by oxygen. Cathode: O2(g) + 4H+(aq) + 4e- 2H2O(l). Anode: Fe(s) Fe2+(aq) + 2e-. Dissolved oxygen in water usually causes the oxidation of iron. Fe2+ initially formed can be further oxidized to Fe3+ which forms rust, Fe2O3.xH2O(s). • Oxidation occurs at the site with the greatest concentration of O2. Preventing Corrosion of Iron • Corrosion can be prevented by coating the iron with paint or another metal. • Galvanized iron is coated with a thin layer of zinc. • Zinc protects the iron since Zn is the anode and Fe the cathode: Zn2+(aq) +2e- Zn(s), Ered = -0.76 V Fe2+(aq) + 2e- Fe(s), Ered = -0.44 V • With the above standard reduction potentials, Zn is easier to oxidize than Fe. Preventing Corrosion of Iron • To protect underground pipelines, a sacrificial anode is added. • The water pipe is turned into the cathode and an active metal is used as the anode. • Often, Mg is used as the sacrificial anode: Mg2+(aq) +2e- Mg(s), Ered = -2.37 V Fe2+(aq) + 2e- Fe(s), Ered = -0.44 V Electrolysis Electrolysis of Aqueous Solutions • Nonspontaneous reactions require an external current in order to force the reaction to proceed. • Electrolysis reactions are nonspontaneous. • In voltaic and electrolytic cells: – – – reduction occurs at the cathode, and oxidation occurs at the anode. In electrolytic cells, electrons are forced to flow from the anode to cathode. Electrolysis – In electrolytic cells the anode is negative and the cathode is positive. (In galvanic cells the anode is positive and the cathode is negative.) • • • • Example, decomposition of molten NaCl. Cathode: 2Na+(l) + 2e- 2Na(l) Anode: 2Cl-(l) Cl2(g) + 2e-. Industrially, electrolysis is used to produce metals like Al. Electroplating • Active electrodes: electrodes that take part in electrolysis. • Example: electrolytic plating. • Consider an active Ni electrode and another metallic electrode placed in an aqueous solution of NiSO4: • Anode: Ni(s) Ni2+(aq) + 2e• Cathode: Ni2+(aq) + 2e- Ni(s). • Ni plates on the inert electrode. • Electroplating is important in protecting objects from corrosion. • • • • • Quantitative Aspects of Electrolysis We want to know how much material we obtain with electrolysis. 1 Ampere is 1 Coulomb per second (A= C/s) 1 mole of electrons = 96,485 C = 1 Faraday Use balanced half-equation to equate moles of substance to moles of electrons Molar mass (in g) = 1 mole of substance Examples 1. A car bumper is to be electroplated with Cr from a solution of Cr3+. What mass of Cr will be applied to the bumper if a current of 0.50 amperes is allowed to run through the solution for 4.20 hours? 2. What volume of H2 gas (at STP) will be produced from the SHE after 2.56 minutes at a current of 0.98 amperes? 3. What volume of F2 gas, at 25°C and 1.00 atm, is produced when molten KF is electrolyzed by a current of 10.0 A for 2.00 hours? What mass of potassium metal is produced? At which electrode does each reaction occur?