Chapter 17-part2
advertisement

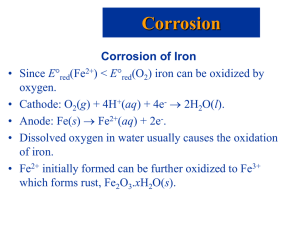
CHAPTER 17 Electrochemistry – part 2 Electrolysis and Electrolytic Cells Anode: where oxidation takes place ◦ Anions are oxidized at this electrode ◦ labeled positive to reflect anions attraction to anode Cathode: where reduction takes places ◦ Cations are reduced at this electrode ◦ Labeled negative to reflect the cations attraction to cathode Electrolysis and Electrolytic Cells • Electrolysis: The process of using an electric current to bring about chemical change. • Process occurring in galvanic cell and electrolytic cells are the reverse of each other • In an electrolytic cell, two inert electrodes are dipped into an aqueous solution Electrolysis and Electrolytic Cells Electrolysis: The process of using an electric current to bring about chemical change. Quantitative Aspects of Electrolysis Charge(C) = Current(A) × Time(s) Moles of e = Charge(C) × 1 mol e 96,500 C Faraday constant Example God can be plated out of a solution containing Au3+ according to the following half-reaction: Au3+(aq) + 3e- Au(s) What mass of gold (in grams) will be plated by the follow of 5.5A of current for 25 minutes? Example Silver can be plated out of a solution containing Ag+ according to the following half-reaction: Ag+(aq) + e- Ag(s) How much time (in minutes) would it takes to plate 12.0 g of silver using a current of 3.0A? Led-acid storage batteries Consists of six cells wired in series. Each cell contains a porous lead anode and a lead oxide cathode, both immersed in sulfuric acid. An electric current is drawn from the battery, both the anode and cathode become coated with PbSO4(s) Can be recharged by running electric current through it in reverse direction Batteries Lead Storage Battery Anode: Cathode: Overall: Pb(s) + HSO4(aq) PbO2(s) + 3H+(aq) + HSO4(aq) + 2e Pb(s) + PbO2(s) + 2H+(aq) + 2HSO41(aq) PbSO4(s) + H+(aq) + 2e PbSO4(s) + 2H2O(l) 2PbSO4(s) + 2H2O(l) Dry-Cell Batteries Zinc acts as the anode and a graphite rod immersed in a moist, slightly acidic pasted of MnO2 and NH4Cl acts a cathode. Anode: Zn(s) + 2OH(aq) ZnO(s) + H2O(l) + 2e Cathode: 2MnO2(s) + H2O(l) + 2e Mn2O3(s) + 2OH(aq) Batteries Dry-Cell Batteries Leclanché cell Anode: Cathode: Zn(s) 2MnO2(s) + 2NH4+(aq) + 2e Zn2+(aq) + 2e Mn2O3(s) + 2NH3(aq)+ H2O(l) Batteries Nickel-Cadmium (“ni-cad”) Batteries Anode: Cathode: Cd(s) + 2OH(aq) NiO(OH)(s) + H2O(l) + e Cd(OH)2(s) + 2e Ni(OH)2(s) + OH(aq) Nickel-Metal Hydride (“NiMH”) Batteries Anode: Cathode: Overall: MHab(s) + OH(aq) NiO(OH)(s) + H2O(l) + e MHab(s) + NiO(OH)(s) M(s) + H2O(l) + e Ni(OH)2(s) + OH(aq) M(s) + Ni(OH)2(s) Batteries Lithium and Lithium Ion Batteries Lithium Anode: xLi(s) Cathode: MnO2(s) + xLi+(soln) + xe xLi+(soln) + xe LixMnO2(s) Lithium Ion Anode: Cathode: LixC6(s) Li1-xCoO2(s) + xLi+(soln) + xe xLi+(soln) + 6C(s) + xe LiCoO2(s) Fuel cells Hydrogen-Oxygen Fuel Cell Like batteries, but the reactants must be constanly replenished. Normal batteries los their ability to generate voltage with use because the reactants become depleted as electric current is drawn from the battery. In fuel cell, the reactant – the fuel-constanly flow through the battery, generating electric current as they undergo redox reaction. Corrosion Corrosion: The oxidative deterioration of a metal. Moisture must be present for rusting to occur Additional electrolytes promote more rusting ◦ Such as NaCl, on the surface of iron because it enhances current flow The presence of acid promotes rusting. (H+ ions are involved in the reduction of oxygen, lower pH enhances the cathodic reaction and leads to faster rusting. Preventing Corrosion Keep dry Coat the iron with a substance that is impervious to water ◦ Painting Placing a sacrificial electrode in electrical contact with the iron. For some metals, oxidation protects the metal (aluminum, chromium, magnesium, titanium, zinc, and others). Corrosion Prevention of Corrosion 1. Galvanization: The coating of iron with zinc. When some of the iron is oxidized (rust), the process is reversed since zinc will reduce Fe2+ to Fe: Fe2+(aq) + 2e Fe(s) Zn2+(aq) + 2e Zn(s) E° = 0.45 V E° = 0.76 V Corrosion Prevention of Corrosion 2. Cathodic Protection: Instead of coating the entire surface of the first metal with a second metal, the second metal is placed in electrical contact with the first metal: Anode: Cathode: Mg(s) O2(g) + 4H+(aq) + 4e Mg2+(aq) + 2e E° = 2.37 V 2H2O(l) E° = 1.23 V Attaching a magnesium stake to iron will corrode the magnesium instead of the iron. Magnesium acts as a sacrificial anode. Molten salt- mixture of cations and anions In general: ◦ The cation that is most easily reduced (the one with least negative, or most positive, reduction-half cell potential) is reduced first ◦ The anion is most easily oxidize ( the one has the least negative, or most positive, oxidation half-cell potential) is oxidized first The cations of active metals-those that are not easily reduced, such as Li+, K+, Na+, Mg2+, Ca2+, and Al3+ - Cannot be reduced from aqueous solution by electrolysis because water is reduced at lower voltage. Electrolysis and Electrolytic Cells Electrolysis of Molten Sodium Chloride Anode: Cathode: Overall: 2Cl(l) 2Na+(l) + 2e 2Na+(l) + 2Cl(l) Cl2(g) + 2e 2Na(l) 2Na(l) + Cl2(g)