Electrochemistry
advertisement

Chemistry 100 – Chapter 20
Electrochemistry
Voltaic Cells
A Schematic Galvanic Cell
e-
Porous Disk
e-
eReducing Agent
Anode
Oxidizing Agent
Cathode
The Galvanic Cell Defined
Galvanic cells – an electrochemical cell that
drives electrons through an external circuit as
a result of the spontaneous redox reaction
occurring inside.
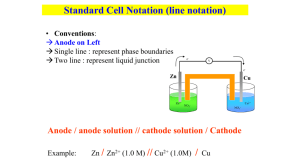
The Zn/Cu Galvanic Cell
Voltaic Cells
We expect the Zn electrode to lose mass and
the Cu electrode to gain mass.
“Rules” of voltaic cells:
At the anode electrons are products. (Oxidation)
At the cathode electrons are reactants (Reduction)
Electrons flow from the anode to the cathode.
The Anode and Cathode
Galvanic cells - the anode is negative
and the cathode is positive.
Electrons are made to flow through an
external circuit. (Rule 3.)
Cell Potentials (Electromotive
Force or EMF Values)
Electromotive force (emf) - aka the cell
potential
the force required to push electrons
through the external circuit.
Ecell is the emf of a cell (old notation).
Now talk about the cell potential!
Cell Reactions
The difference in the RHS and the LHS
reaction
Cu2+ (aq) + Zn (s) Cu (s) + Zn2+ (aq)
For each half reaction, we can write the
reaction quotient (see Chapter 15) as follows
Cu2+ (aq) + 2 e- Cu (s) Q = 1/ [Cu2+]
Zn2+ (aq) + 2 e- Zn (s)
Q = 1/ [Zn2+]
Overall Qcell = [Zn2+] / [Cu2+]
The Cell Potential and G
From the reaction Gibbs energy
rxn G rxn G RT ln Q cell F E cell
o
rxn G rxn G o RT ln Qcell
E cell
F
F
F
The Nernst Equation
E cell
RT
E
ln Q cell
F
E - standard cell
potential
Cell potential under
standard conditions.
[Solutes] = 1.000 mole/L
T = 298.15 K
P = 1.00 atm pressure
E cell
rxn G
F
o
Cell Potentials
Standard Reduction Potentials
We cannot measure the potential of an
individual half-cell!
We assign a particular cell as being our
reference cell and then assign values to
other electrodes on that basis.
Cell Potentials are Intensive
Properties
In the previous example, the cell potential
was simply the difference between the
standard potential for the Sn4+/Sn2+ reduction
and the Fe3+/Fe2+ reduction.
Reason: standard cell potentials are intensive
quantities.
E cell
rxn G
F
E cell
rxn G o
F
The Standard Hydrogen
electrode
Eo (H+/H2) half-cell = 0.000 V
ep{H2(g)} = 1.00 atm
H2 (g)
[H+] = 1.00
Pt gauze
A Galvanic Cell With Zinc and the
Standard Hydrogen Electrode.
Note - [Zn2+]= [H+] = 1.000 M
The Cell Equation for the ZincStandard Hydrogen Electrode.
The cell reaction
2 H+ (aq) + Zn (s) H2 (g) + Zn2+ (aq)
When we measure the potential of this cell
Ecell = ERHS - ELHS
but ERHS = E(H+/H2) = 0.000 V
Ecell = E(Zn2+/Zn) = -0.763 V
The Spontaneous Direction of
a Cell reaction
Examine the magnitude the of the
standard cell potential!
o
rxn G
E cell
F
If Eo is positive, the rG is negative! Under
standard conditions, the cell will proceed
spontaneously in the direction written for the
cell reaction.
The Composition Dependence
of the Cell Potential
Nernst equation
the nonstandard cell potential (Ecell) will be
a function of the concentrations of the
species in the cell reaction.
E cell
RT
E
ln Q cell
F
To calculate Ecell, we must know the cell
reaction and the value of Qcell.
Electrochemical Series
Look at the following series of reactions
Cu2+ (aq) + 2 e- Cu (s) E(Cu2+/Cu) = 0.337 V
Zn2+ (aq) + 2 e- Zn (s) E(Zn2+/Zn) = -0.763 V
Zn has a thermodynamic tendency to reduce
Cu2+ (aq)
Pb2+ (aq) + 2 e- Pb (s) E(Pb2+/Pb) = -0.13 V
Fe2+ (aq) + 2 e- Fe (s) E(Fe2+/Fe) = -0.44 V
Fe has a thermodynamic tendency to reduce
Pb2+ (aq)
Differences in Reduction
Potentials
• The larger the
difference between
Ered values, the larger
Ecell.
• In a voltaic (galvanic)
cell (spontaneous)
Ered(cathode) is more
positive than
Ered(anode).
Oxidizing and Reducing
Agents
The more positive Ered the stronger the
oxidizing agent on the left.
The more negative Ered the stronger
the reducing agent on the right.
Spontaneous Oxidation
Processes
A species on the higher to the left of
the table of standard reduction
potentials will spontaneously oxidize a
species that is lower to the right in the
table.
Any species on the right will
spontaneously reduce anything that is
higher to the left in the series.
Oxidizing and Reducing
Agents
Concentration Cells
Two identical half-cells.
RHS
LHS
AgCl (s) + e- Ag (s) + Cl- (aq, 0.10 M)
AgCl (s) + e- Ag (s) + Cl- (aq, 0.50 M)
Electrolyte concentration cell – the
electrodes are identical; they simply
differ in the concentration of electrolyte
in the half-cells.
The Nernst equation for the cell
RT
E cell
ln Q cell
F
RT [Cl ]RHS
ln
F [Cl ] LHS
Cells at Equilibrium
When the electrochemical cell has
reached equilibrium
E cell 0 V Qcell K cell
Kcell = the equilibrium constant for the cell reaction.
RT
FE
E
ln K cell ln K cell
F
RT
Knowing the E° value for the cell, we can estimate
the equilibrium constant for the cell reaction.
Equilibrium Constants from
Cell Potentials
Examine the following cell.
Half-cell reactions.
Sn4+ (aq) + 2 e- Sn2+ (aq)
= 0.15 V
Fe3+ (aq) + e- Fe2+ (aq)
= 0.771 V
E(Sn4+/Sn2+)
E (Fe3+/Fe2+)
Cell Reaction
Sn4+ (aq) + 2 Fe3+ (aq) Sn2+ (aq) + 2 Fe2+
(aq)
Ecell = (0.771 - 0.15 V) = 0.62 V
Lead-Acid Battery
A 12 V car battery - 6 cathode/anode
pairs each producing 2 V.
Cathode: PbO2 on a metal grid in sulfuric
acid:
PbO2(s) + SO42-(aq) + 4H+(aq) + 2e-
PbSO4(s) + 2H2O(l).
Anode: Pb:
Pb(s) + SO42-(aq) PbSO4(s) + 2e-
Lead-Acid Battery
The overall electrochemical reaction is
PbO2(s) + Pb(s) + 2SO42-(aq) + 4H+(aq)
2PbSO4(s) + 2H2O(l)
for which
Ecell = ERHS - ELHS
= (+1.685 V) - (-0.356 V)
= +2.041 V.
Wood or glass-fiber spacers are used to
prevent the electrodes form touching.
A Picture of a Car Battery
An Alkaline Battery
Anode: Zn cap:
Zn(s) Zn2+(aq) + 2e Cathode: MnO2, NH4Cl and carbon paste:
2 NH4+(aq) + 2 MnO2(s) + 2e- Mn2O3(s) +
2NH3(aq) + 2H2O(l)
Graphite rod in the center - inert cathode.
Alkaline battery, NH4Cl is replaced with KOH.
Anode: Zn powder mixed in a gel:
The Alkaline Battery
Fuel Cells
Direct production of electricity from
fuels occurs in a fuel cell.
H2-O2 fuel cell was the primary source
of electricity on Apollo moon flights.
Cathode: reduction of oxygen:
2 H2O(l) + O2(g) + 4e- 4OH-(aq)
Anode:
2H2(g) + 4OH-(aq) 4H2O(l) + 4e-
Fuel Cells
Corrosion of Iron
Since E(Fe2+/Fe) < E(O2/H2O) iron can be
oxidized by oxygen.
Cathode
Anode
O2(g) + 4H+(aq) + 4e- 2H2O(l).
Fe(s) Fe2+(aq) + 2e-.
Fe2+ initially formed – further oxidized to Fe3+
which forms rust, Fe2O3• xH2O(s).
Rusting (Corrosion) of Iron
Preventing the Corrosion of
Iron
Corrosion can be prevented by coating
the iron with paint or another metal.
Galvanized iron - Fe is coated with Zn.
Zn protects the iron (Zn - anode and Fe
- the cathode)
Zn2+(aq) +2e- Zn(s), E(Zn2+/Zn) = -0.76 V
Fe2+(aq) + 2e- Fe(s), E(Fe2+/Fe) = -0.44 V
Preventing the Corrosion of
Iron
Preventing the Corrosion of
Iron
To protect underground pipelines, a
sacrificial anode is added.
The water pipe - turned into the
cathode and an active metal is used as
the sacrificial anode.
Mg is used as the sacrificial anode:
Mg2+(aq) +2e- Mg(s), E(Mg2+/Mg) = -2.37 V
Fe2+(aq) + 2e- Fe(s), E(Fe2+/Fe) = -0.44 V
Corrosion Prevention
Electrolysis of Aqueous
Solutions
Nonspontaneous reactions require an
external current in order to force the
reaction to proceed.
Electrolysis reactions are nonspontaneous.
In voltaic and electrolytic cells:
reduction occurs at the cathode, and
oxidation occurs at the anode.
Voltaic vs.Electrolytic Cells
Electrolytic cells – electrons are forced
to flow from the anode to cathode.
In electrolytic cells the anode is positive
and the cathode is negative. (In
galvanic cells the anode is negative and
the cathode is positive.)
Electrolysis of Aqueous
Solutions
Electrolysis of Molten Salts
Decomposition of molten NaCl.
Cathode: 2Na+(l) + 2e- 2Na(l)
Anode: 2Cl-(l) Cl2(g) + 2e-.
Industrially, electrolysis is used to
produce metals like Al.
Electrolysis With Active
Electrodes
Active
electrodes:
electrodes
that take
part in
electrolysis.
Example:
electrolytic
plating.
Electrolysis With Active
Electrodes (cont’d)
Consider an active Ni electrode and
another metallic electrode placed in an
aqueous solution of NiSO4:
Anode: Ni(s) Ni2+(aq) + 2e Cathode: Ni2+(aq) + 2e- Ni(s).
Ni plates on the inert electrode.
Electroplating is important in protecting
objects from corrosion.
Quantitative Aspects of
Electrolysis
Consider the reduction of Cu2+ to Cu.
Cu2+(aq) + 2e- Cu(s).
2 mol of electrons 1 mol of Cu. How
much material is obtained?
Q=It
current (I)
time (t) of the plating process.
Gibbs Energy and Work
Gibbs energy – the
maximum amount
of useful work that
can be obtained
from a system.
G w max
G nFE
w max nFE
Note – if wmax is negative, then work is
performed by the system and E is
positive.
Electrical Work
Eelectrolytic cell – external source of
energy is required to force the reaction
to proceed.
External emf must be greater than Ecell.
From physics: work has units watts.
1 W = 1 J/s.
Units of Electrical Work
Electric utilities use units of kilowatthours:
3600 s 1 J/s
1 kWh 1000 W 1 h
1 h 1 W
6
3.6 10 J.