Learning Targets
advertisement

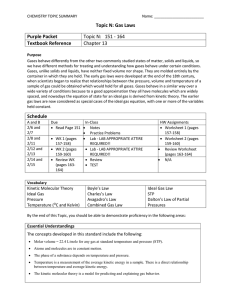
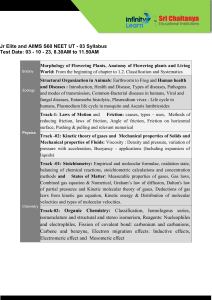
Learning Targets - Unit 6 Gases 1. Define, apply, and distinguish among the following key terms: absolute temperature direct relationship pressure inverse relationship barometer kinetic energy mm of Hg (Torr) kinetic molecular theory atmosphere (atm) elastic collision kilopascal (kPa) ideal gas law vacuum ideal gas constant standard temperature & pressure (STP) absolute zero Kelvin molar volume 2. Distinguish between the characteristics of solids, liquids, and gases. 3. State the mathematical relationships between the measurable properties of gases: pressure, volume, temperature, and number of moles 4. Explain the characteristics and behavior of gases using Kinetic Molecular Theory. 5. Apply gas properties and behavior to explain real life examples. 6. Define pressure, give the SI unit for pressure, and convert between standard units of pressure 7. Convert temperatures between units of Celsius and Kelvin. 8. Use the Combined gas law equation to calculate P, V, n, or T. 9. Define Standard Temperature and Pressure. (STP) 10. Describe the properties of an Ideal Gas. 11. Solve problems using the Ideal gas law equation.