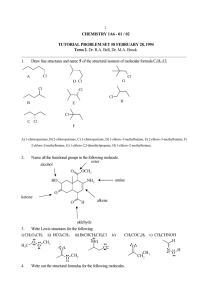
(c) cycloalkanes that do not show cis,trans isomerism.
advertisement

5 Organic Chemistry William H. Brown & Christopher S. Foote 5-1 5 Alkenes I Chapter 5 5-2 5 Unsaturated Hydrocarbons Unsaturated hydrocarbon: contains one or more carbon-carbon double or triple bonds Alkene: contains a carbon-carbon double bond and has the general formula CnH2n H H C C H H Ethylene (an alkene) Alkyne: contains a carbon-carbon triple bond and has the general formula CnH2n-2 H-C C-H Acetylene (an alkyne) . 5-3 5 Unsaturated Hydrocarbons Arenes: benzene and its derivatives (Ch 20-21) H H H C C C C C C H H H 5-4 5 Benzene & Phenyl Group We do not study benzene and its derivatives until Chapters 20 & 21 • but, we show structural formulas of compounds containing the phenyl group before that time • the phenyl group is not reactive under any of the conditions we describe in Ch 6-19 C6 H5 Benzene Ph- Alternative representations for the phenyl group 5-5 5 Structure of Alkenes The two carbon atoms of a double bond and the four atoms attached to them lie in a plane, with bond angles of approximately 120° A double bond consists of • one sigma bond formed by overlap of sp2 hybrid orbitals • one pi bond formed by overlap of parallel 2p orbitals • it takes approx. 264 kJ (63 kcal)/mol to break the pi bond in ethylene 5-6 5 Restricted rotation about C=C QuickTime™ and a Photo - JPEG decompressor are needed to see this picture. 5-7 5 Cis,Trans Isomerism Cis,trans isomers: isomers that have the same connectivity but a different arrangement of their atoms in space due to the presence of a ring or a carbon-carbon double bond H H C H3 C H C CH 3 C CH3 cis-2-Butene mp -139°C, bp 4°C H3 C C H trans-2-Butene mp -106°C, bp 1°C 5-8 5 IUPAC Nomenclature 1. Number the longest chain of carbon atoms that contains the double bond in the direction that gives the carbons of the double bond the lowest numbers 2. Locate the double bond by the number of its first carbon 3. Name substituents 4. Number the carbon, locate and name substituents, locate the double bond, and name the main chain. 6 5 4 3 2 1-Hexene 1 6 54 3 2 1 4-Methyl-1-hexene 5 4 32 1 2-Ethyl-4-methyl-1-pentene 5-9 5 Alkenyl Groups Alkenyl Group Common Name Example CH2 = methylene CH2 methylenecyclohexane CH2 = CH- vinyl CH2 = CHCl vinyl chloride CH2 = CHCH 2 - allyl CH2 = CHCH2 Cl allyl chloride 5-10 5 Common Names Despite the precision and universal acceptance of IUPAC nomenclature, some alkenes, particularly low-molecular-weight ones, are known almost exclusively by their common names CH3 CH2 = CH2 IUPAC: Ethene Common: Ethylene CH3 CH= CH 2 CH3 C= CH2 Propene 2-Methylpropene Propylene Isobutylene 5-11 5 Configuration The cis,trans system: configuration is determined by the orientation of atoms of the main chain H CH2 CH3 C CH3 CH 2 C H 1 H trans-3-Hexene H3 C 2 C 3 CH3 C 4 CH( CH 3 ) 2 cis-3,4-Dimethyl-2-pentene 5-12 5 Configuration - E,Z • uses priority rules (Chapter 3) • if groups of higher priority are on the same side, configuration is Z (German, zusammen) • if groups of higher priority are on opposite sides, configuration is E (German, entgegen) higher higher C lower higher C lower C lower Z (zusammen) lower C higher E (entgegen) 5-13 5 Configuration - E,Z Example: name each alkene and specify its configuration by the E,Z system Cl (a) (b) Cl (c) (d) Cl Br 5-14 5 Cis,Trans Isomerism Cycloalkenes • configuration of the double bond in cyclopropene through cycloheptene must be cis • cyclopentene is only slightly puckered 5-15 5 Cis,Trans Isomerism • the configuration of the double bond in cyclohexene must be cis • cyclohexene is puckered 5-16 5 Cis,Trans Isomerism Cycloalkenes • trans-cyclooctene is the smallest trans-cycloalkene stable at 25°C • the cis isomer is 38 kJ (9.1 kcal)/mol more stable than the trans isomer. trans-Cyclooctene (a pair of enantiomers) 5-17 5 Physical Properties Alkenes are nonpolar compounds The only attractive forces between their molecules are dispersion forces The physical properties of alkenes are similar to those of alkanes 5-18 5 Terpenes Terpene: a compound whose carbon skeleton can be divided into two or more units identical with the carbon skeleton of isoprene head 1 2 3 4 tail 2-Methyl-1,3-butadiene (Isoprene) 5-19 5 Terpenes Myrcene, C10H16, a component of bayberry wax and oils of bay and verbena Menthol, from peppermint OH 5-20 5 Terpenes • -Pinene, from turpentine • camphor, from the camphor tree O 5-21 5 Terpenes Vitamin A (retinol) • how many stereoisomers are possible for this terpene alcohol? OH 5-22 5 Prob 5.7 Using the VSEPR model, predict all bond angles about each circled atom. (a) (c) (b) O C-OH CH 2 OH (d) (e) HC C-CH= CH 2 5-23 5 Prob 5.12 Name these alkenes and cycloalkenes. Cl (a) (b) (c) Cl (e) (d) (g) (h) F F F (f) Cl Cl F (i) 5-24 5 Prob 5.13 Arrange the groups in each set in order of increasing priority. (a) - CH 3 (b) - OCH 3 (c) - CH 3 -H -Br -CH 2 CH 3 -CH( CH3 ) 2 -CH 2 OH -B( CH 2 CH 3 ) 2 -CH 2 NH 2 -H -CH 2 Br 5-25 5 Prob 5.14 Assign an E or Z configuration to each compound. Also assign a cis or trans configuration to each. H COOH C (a) H OOC C H OOC (b) H Fumaric acid COOH C H C CH2 COOH Aconitic acid 5-26 5 Prob 5.16 For each molecule that shows cis,trans isomerism, draw the cis isomer. CH3 (a) CH3 CH3 (b) CH3 CH3 CH3 (d) (c) CH3 CH3 5-27 5 Prob 5.17 Draw structural formulas for all compounds of molecular formula C5H10 that are (a) alkenes that do not show cis,trans isomerism. (b) alkenes that do show cis,trans isomerism. (c) cycloalkanes that do not show cis,trans isomerism. (d) cycloalkanes that do show cis,trans isomerism. 5-28 5 Prob 5.19 Draw a structural formula of a bromoalkene of molecular formula C5H9Br that shows (a) neither E,Z isomerism nor chirality. (b) E,Z isomerism but not chirality. (c) chirality but not E,Z isomerism. (d) both chirality and E,Z isomerism. 5-29 5 Prob 5.23 Which alkenes exist as pairs of cis,trans isomers? For each that does, draw the trans isomer. (a) CH2 = CHBr (b) CH3 CH= CHBr (c) BrCH= CHBr (d) ( CH3 ) 2 C= CHCH3 (e) ( CH3 ) 2 CH CH = CH CH3 5-30 5 Prob 5.24 Show that four stereoisomers are possible for this compound. Draw the stereoisomer with an E configuration at the double bond and an R configuration at the stereocenter. OH CH3 -CH= CH-CH- CH3 3-Penten-2-ol 5-31 5 Prob 5.28 Show that warburganal is a terpene. Label all stereocenters and state how many stereoisomers are possible for a molecule of this structure. Warburganal []25 D -260 CHO H3 C OH CHO H H3 C CH3 5-32 5 Prob 5.29 Show that santonin is a terpene. Label all stereocenters and tell how many stereoisomers are possible for the molecule of this structure. CH3 CH3 O CH3 O O 5-33 5 Prob 5.30 (a) Show that zoapatanol is a terpene. (b)Specify the configuration at the double bond to the seven-membered ring. (c) How many stereoisomers are possible for this molecule? OH O HO O 5-34 5 Prob 5.31 Pyrethrin II Label all stereocenters in pyrethrin II. How many stereoisomers are possible for this molecule? O H 3 COC H CH3 O H H3 C H CH3 O C O H CH3 5-35 5 Prob 5.31 Pyrethrosin Show that pyrethrosin is a terpene. Label all stereocenters in pyrethrosin and state how many stereoisomers are possible for it. H O H O O CH3 CH 3 O H CH 2 CCH 3 O 5-36 5 Alkenes I End Chapter 5 5-37