Unit: Chemical Interactions Chapter 8: Solutions When substances
advertisement
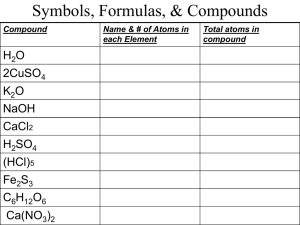
Unit: Chemical Interactions Chapter 8: Solutions When substances dissolve to form solutions, the properties of the mixture change. 8.1: A solution is a type of mixture 8.2: The amount of solute that dissolves can vary 8.3: Solutions can be acidic, basic, or neutral 8.4: Metal alloys are solid mixtures 8.1: A solution is a type of mixture Review / warm-up What are their chemical formulas? What are the elements? Which is an ionic compound and which is a covalent compound? Are the properties of each compound the same/different than the atoms/elements that form them? What is the different in how these compounds are held together? C Na Cl O animation O 8.1: A solution is a type of mixture 8.1 A solution is a type of mixture 8.1: A solution is a type of mixture The parts of a solution are mixed evenly Mixture: a combination of substances – ex: fruit salad, chili Can physically separate ingredients because they are not chemically changed – still the same substances If a mixture is so completely blended together… The ingredients canNOT be separated and identified as different substances Solution: a type of mixture, called a homogeneous mixture “same throughout” (“well-mixed”) All portions of the the mixture have the same properties Sand + Water Sand sinks to the bottom ; Solution? Sugar + water sugar is dispersed throughout; Solution? Other common solutions: seawater, gasoline, liquid part of blood 8.1: A solution is a type of mixture Solutes and Solvents – components of a solution Solutions – have a definite composition Solute – a substance that is dissolved to make a solution When it dissolves, it separates into individual particles It is dissolved into the… Solvent – a substances that dissolves a solute Most common: water Others – ex: turpentine, soaps - to remove oils After the solute dissolves and separates into individual particles, it is not possible to identify the solute and solvent as different substances Ex: Solution Salt water blood Solute salt Calcium ions, sugar solvent water water 8.1: A solution is a type of mixture A salt water solution Solute (salt) Solvent (water) animation 8.1: A solution is a type of mixture Types of Solutions Gas Solution: Ex: air = oxygen (an other gases) dissolved in nitrogen Liquid Solution: Ex: vinegar = acetic acid dissolved in water Solid Solution: Ex: bronze = tin dissolved in copper Must first be melted to a liquid, then mixed, then cooled to solid Mixed? Ex: soda = carbon dioxide dissolved in water solution Ex: saltwater = salt dissolving in water Solution Worksheet solute solvent 8.1: A solution is a type of mixture Suspensions Suspension: the particles added are larger than those found in a solution, so instead of dissolving, these larger particles turn the liquid cloudy Ex: Flour added to water Can sometimes separate the components of the suspension using a filter 8.1: A solution is a type of mixture Solvent and solute particles interact The parts of a solution are not changed into new substances The solute and the solvent can still be physically separated, though they do interact Ex: a solid dissolves in a liquid, the particles of the solute are surrounded by particles of the liquid (solvent) The solute particles become evenly distributed through the solvent 8.1: A solution is a type of mixture Solvent and solute particles interact (2) The way a solid compound dissolves in a liquid depends on the type of bonds in the compound Ionic compounds split apart into individual ions Ex: table salt dissolves in water – the sodium and chloride ions separate, and each ion is surrounded by water molecules Covalent compounds dissolved in water, the molecules stay together and are surrounded by solvent molecules Ex: table sugar dissolves in water – C12H22O11 stays as such Properties of solvents change in solutions Solutes change the physical properties of a solvent in every solution A solution’s physical properties differ from the physical properties of the pure solvent Dependent on the amount of solute added Lowering the Freezing Point Freezing Point: temperature Liquid Solid A solvent’s freezing point is lowered when a solute is dissolved in it Ex: add salt to water – freezing point drops below 32oF (0oC) Useful for snow and ice on sidewalks and roads (water freezes at a lower temperature, can help to melt ice) • A limit: can get down to -6oF (-21oC) before the melted ice will freeze again • Ice cream maker: • Canister surrounding ingredients holds a mixture of salt and ice • The lower freezing point causes the ice to melt, absorbing heat from surroundings • This includes the ice cream ingredients, which get chilled • Then tiny ice crystals form all at once in the ice cream mixture rather than a few crystals growing larger over time (as would happen in a regular freezer) Raising the Boiling Point Boiling Point: temperature liquid gas Boiling point of a solution is higher than the boiling point of the pure solvent Solution can remain a liquid at a higher temperature than its pure solvent Ex: boiling point of water = 212oF (100oC) Salt raises the boiling point of water Dependent on amount of salt added Summary: a solute lowers the freezing point and raises the boiling point of the solvent in the solution Extends the temperature range in which the solvent remains a liquid Uses: antifreeze in a car’s radiator (ethylene glycol added to water) Prevents the water from freezing in the winter Keeps it from boiling in the summer s