Matter Classification
advertisement

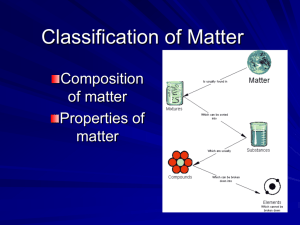
Bellringer: What is the name of the temperature for when a solid changes into a liquid? Bellringer: Why would air be considered a mixture? Think about what air is composed of. Today’s objectives: Review types of matter. Describe and illustrate the particle arrangement of matter. Classify matter according to its composition and physical properties. Recall, that we learned one physical property used to describe matter is color. The color of the sunset depends on how many particles are mixed in the air to scatter the light. What do you think these particles in the air come from? Pollution, dust, water vapor, etc. As the Sun gets lower in the sky, its light passes through more of the atmosphere to reach you. Even more of the blue and violet light is scattered, allowing the reds and yellows to pass straight through to your eyes without all that competition from the blue and violet colors. Large particles of dust, pollution, and water vapor in the atmosphere reflect and scatter more of the reds and yellows, sometimes making the whole western sky glow red. The fact that the air particles scatter light is another physical property of certain groups of matter. Today’s objectives: We are going to Review the types of matter. Previously we discussed the classification of matter based on its composition. What were these two main types of matter? Type of matter: Substances Properties of this type of matter: 1. Combined Chemically 2. Can not be broken down Physically 3. Considered to be “pure” matter Type of matter: Mixtures Properties of this type of matter: 1. Combined Physically 2. Can be broken down Physically 3. Considered to be “impure” matter Can you tell us the two types of substances? The 2 subgroups of substances are Compounds ________________ and ________________. Elements Can you tell us the two types of mixtures? The 2 subgroups of substances are Heterogeneous ________________ Homogeneous and ________________. Organize our thoughts into the following graphic organizer: Matter Substances Mixtures 2 Types Element Would be found on the periodic table. 2 Types Compound Two or more elements chemically combined. Has a chemical formula. Homogeneous Particles are even mixed throughout. Contains particles that are not visible. Heterogeneous Particles are NOT evenly mixed throughout. Contains particles that are visible. Another name for this group: 2 Types Solution Colloid Suspension This is called the Tyndall effect. Does not settle if left standing still. Light Test shows: light goes through Light Test shows: light scatters Settles if left standing still. Today’s objectives: the types of matter. Now we will Classify matter according to its composition and physical properties. We are going to be viewing samples of matter. Then we will classify these samples based on what you know. When we determine the appropriate place for the sample, write the name of the sample in the appropriate block on the line provided. DO NOT GLUE PICTURES UNTIL THE END! Pull up screen to write on board, call on students to complete activity Substances Mixtures Homogeneous Mixture Element Compound Solution _______ ________ ________ Heterogeneous Mixtures Dry Heterogeneous Mixture ________ Colloid ________ Suspension ________ Substances Mixtures Homogeneous Mixture Element Compound Solution Fruit Aluminum ________ Salt _______ ________ juice Heterogeneous Mixtures Dry Heterogeneous Mixture Beads ________ Colloid Wax ________ Suspension Oil & ________ water Quietly and quickly pair up with the person to your right. Move your desks close together. Substances Mixtures Now glue your pictures onto your paper ! Homogeneous Mixture Element Compound Solution Fruit Aluminum ________ Salt _______ ________ juice Heterogeneous Mixtures Dry Heterogeneous Mixture Beads ________ Colloid Wax ________ Suspension Oil & ________ water Now you try. Classify the following examples. Write the name of the samples in the correct location on your paper. Fog Italian dressing Mercury Water Bird seed Brass Substances Mixtures Lets see if you were correct Homogeneous Mixture Element Compound Solution Mercury ________ Water Brass _______ ________ Heterogeneous Mixtures Dry Heterogeneous Mixture Bird seed ________ Colloid Fog ________ Suspension Italian ________ dressing Quickly and quietly move your desks apart. Today’s objectives: Review types of matter. Describe and illustrate the particle arrangement of matter. Classify matter according to its composition and physical properties. We will draw and describe the particles in certain samples of matter. These samples are what phases of matter? Fog Fruit juice Beads Draw and briefly describe the particle arrangement of each phase of matter. Fog Fruit juice Beads For instance particle arrangement of the beads would look like this…. Particles are closely packed together. Beads Now you draw and briefly describe the particle arrangement of the fog and the juice. Fog Fruit juice Conclusion for Today’s Activity: Answer the quiz questions on the last page of your packet. Make sure your name is on the front. Turn in entire packet when you are finished. Exit for Today: Put your name on the front of the index card. On the front of the index card is a letter. Select a word starting with that letter that relates to today’s topic. On the front of the card write and define the word. On the back draw an illustration of the word.