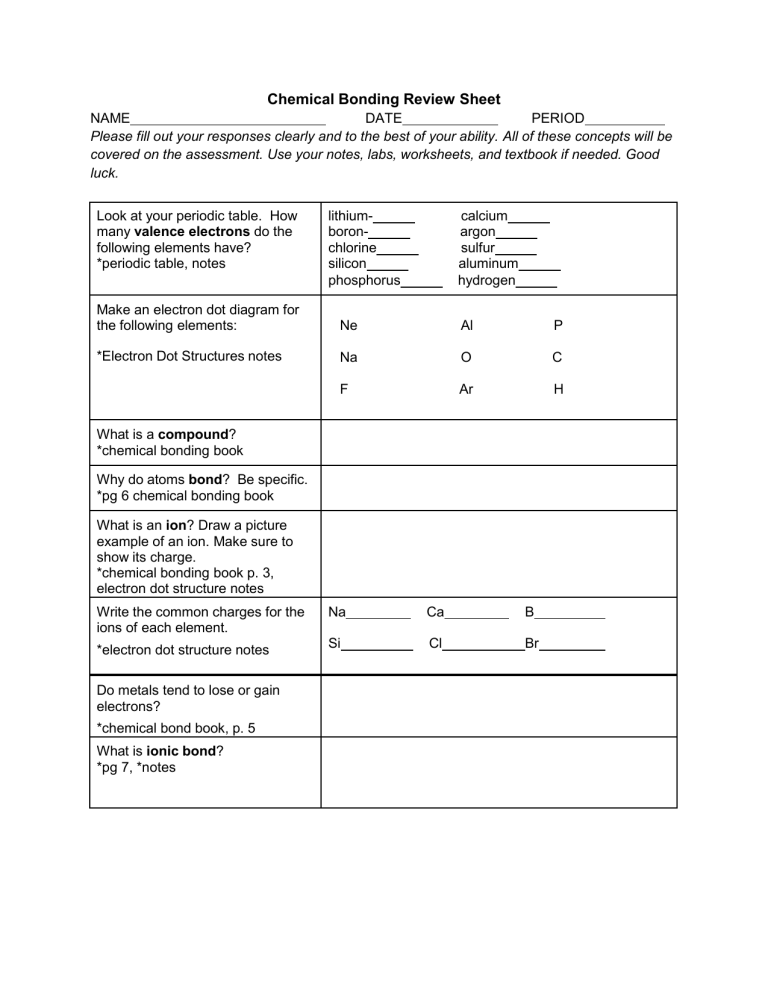
Chemical Bonding Review Sheet NAME DATE PERIOD Please fill out your responses clearly and to the best of your ability. All of these concepts will be covered on the assessment. Use your notes, labs, worksheets, and textbook if needed. Good luck. Look at your periodic table. How many valence electrons do the following elements have? *periodic table, notes lithiumboronchlorine silicon phosphorus calcium argon sulfur aluminum hydrogen Make an electron dot diagram for the following elements: Ne Al P *Electron Dot Structures notes Na O C F Ar H What is a compound? *chemical bonding book Why do atoms bond? Be specific. *pg 6 chemical bonding book What is an ion? Draw a picture example of an ion. Make sure to show its charge. *chemical bonding book p. 3, electron dot structure notes Write the common charges for the ions of each element. Na Ca B *electron dot structure notes Si Cl Br Do metals tend to lose or gain electrons? *chemical bond book, p. 5 What is ionic bond? *pg 7, *notes Write the following ionic compound (chemical formula) that results from the bonding pairs. Then on the line, write the name of the compound. a) Potassium + Iodine Chemical name:____________________________ b) Magnesium+ Iodine *Ionic Bonding Notes page (criss cross method), periodic table, electron dot/charge notes Chemical name:______________________ c) Aluminum + Fluorine Chemical name:________________________ What is a covalent bond? *Chemical bond book, p. 8 What types of elements usually go through covalent bonding? *pg 8 Name 5 properties of ionic compounds. 12- *Lab sheet, notes, pg 701 345Name 5 properties of covalent compounds. 12- *Lab sheet, notes, pg 701 345- Write ionic or covalent for each compound *notes N2O4 KF F2 KCl H2O MgBr2 Name the following ionic compounds *notes MgO2 NaCl Na2O K2S Name the chemical formula for each compounds Ionic (make sure you balance out the charges) Sodium and chlorine calcium and chlorine aluminum and chlorine *notes, practice sheets Covalent: 1 carbon + 1 chlorine 1 nitrogen + 4 oxygen 3 hydrogen +1 nitrogen



