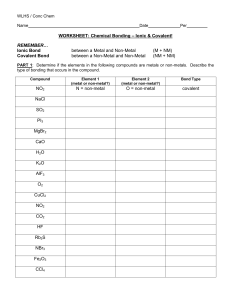
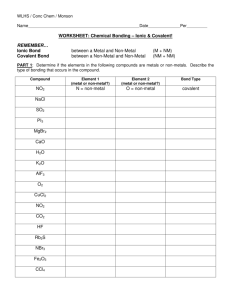
Name WORKSHEET: Chemical Bonding – Ionic & Covalent! REMEMBER… Ionic Bond Covalent Bond between a Metal and Non-Metal between a Non-Metal and Non-Metal (M + NM) (NM + NM) PART 1: Determine if the elements in the following compounds are metals or non-metals. Describe the type of bonding that occurs in the compound. Name each compound accordingly. Compound Element #1 metal or nonmetal? Element #2 metal or nonmetal? Bond Type PO2 P = non-metal O = non-metal covalent CsCl SO2 PI3 MgBr2 K2O AlF3 HF PART 2: Use Lewis dot structures to show the ionic bonding in the following pairs of elements. Show the transfer of electrons using arrows. Write the correct chemical formula for the ionic compound that forms. 1) barium chloride (Ba and Cl) 3) sodium oxide (Na and O) 2) calcium chloride (Ca and Cl) 4) sodium nitride (Na and N) PART 3: Use Lewis dot structures to show the covalent bonding in the following pairs of elements. Use a dash to represent a shared pair of electrons, and dots to show unshared electrons. 1) nitrogen triiodide (NI3) Final Answer: Show work here…HINT: nitrogen is in the middle! 2) carbon tetrabromide (CBr4) Show work here…HINT: carbon is in the middle! 3) dihydrogen monoxide (H2O) Show work here..HINT: oxygen is in the middle! PART 4: Name the following oxyacids using the correct suffix and the polyatomic ion table. Ex. H(ClO4) = Hydrogen + Perchlorate - perchloric acid 1. H2(SO4) 2. H3(PO4) 3. H(ClO2) 4. H2(CrO4) 5. H(NO2) PART 5: Answer the following questions. 1. How are ionic bonds and covalent bonds different? 2. Describe the relationship between the length of a bond and the strength of that bond.