Specific Heat & Calorimetry Presentation
advertisement

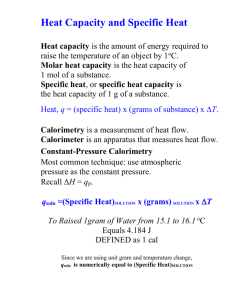
10.3.1 Specific Heat and Calorimetry Date, Section, Pages, etc. Mr. Richter Agenda Check and Review HW Warm Up Intro to Specific Heat Notes: Specific Heat Capacity Calorimetry Practice Problems Objectives: We Will Be Able To… Define specific heat capacity. Perform calculations with specific heat capacity. Understand the process of calorimetry and perform calorimetry calculations. Warm-Up: When you go to the beach in the summer, the sand is often much hotter than the adjacent water. Why is this? Discuss at your table, and we will discuss as a class in a few minutes. Specific Heat Capacity Specific Heat Capacity All substances have different molecular structures due to either: different elements, or different arrangements of atoms As a result, different substances require different amounts of energy to raise their temperatures. Generally, a substance’s specific heat capacity is the amount of energy required to raise the temperature of an object. Specific Heat Capacity What factors will determine how much energy is required to raise the temperature of a substance? the mass (how much) the difference in temperature “Specifically”: specific heat capacity is the quantity of energy needed to raise the temperature of 1 kg of a substance by 1 °C (at constant pressure) Units: J kg oC Specific Heat Capacity p.372 Specific Heat Capacity Q = Cp mDT Specific Heat Capacity: Practice Problem How much energy is required to raise the temperature of 15.0 kg of water by 20.0 °C? Q = (4186)(15.0)(20.0) Q = 1.26 x 106 J Calorimetry How is specific heat determined? Calorimetry The specific heat capacity of water has been studied extensively and is well known. Cp,water = 4186 J/kg °C Scientists can use the principles of calorimetry and the specific heat capacity of water to determine the specific heat capacities of other substances. Calorimetry is the experimental procedure used to measure the energy transfer from one substance to another as heat. Calorimetry: How it works Place an object with a known mass known temperature unknown specific heat Into a container of water with: a known mass known temperature known specific heat When these reach thermal equilibrium, you will know: how much energy the water absorbed, which is equal to… how much energy the object lost From this you can determine the specific heat capacity of the substance. Calorimetry Heat absorbed by water = heat released by object. Qw = Qx c p,w mw DTw = cp,x mx DTx Practice Problem A 0.050 kg metal bolt is heated to an unknown initial temperature. It is then dropped into a beaker containing 0.15 kg of water with an initial temperature of 21.0°C. The bolt and the water then reach a final temperature of 25.0°C. If the metal has a specific heat capacity of 899J/kg °C, find the initial temperature of the metal. Hint: find the change in temperature of the metal first (solve for ΔTx, then find the initial temp. Wrap-Up: Did we meet our objectives? Define specific heat capacity. Perform calculations with specific heat capacity. Understand the process of calorimetry and perform calorimetry calculations. Homework Due Monday: p374 #4, 7