Hints on Column Chromatography
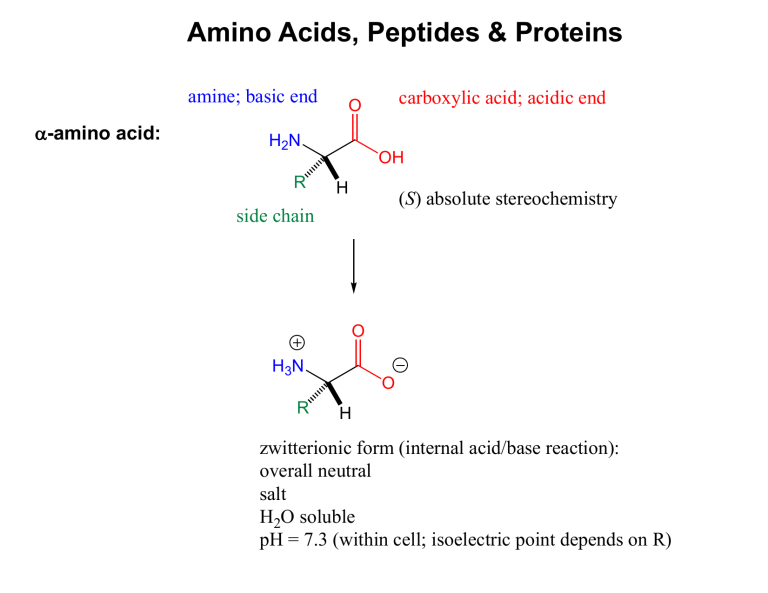
Amino Acids, Peptides & Proteins
a
-amino acid: amine; basic end
H
2
N
R side chain
H
O
OH carboxylic acid; acidic end
( S ) absolute stereochemistry
O
H
3
N
O
R
H zwitterionic form (internal acid/base reaction): overall neutral salt
H
2
O soluble pH = 7.3 (within cell; isoelectric point depends on R)
Amino Acids
• Are >500 naturally occurring amino acids identified in living organisms
O
H
2
N
OH
H
H
Glycine (achiral)
• Humans synthesize
10 of the 20 they use.
The other 10 are called essential amino acids.
H
2
N
H
2
NH
2
CH
2
CH
2
CH
2
C
H
O
OH
Lysine (basic, nucleophilic, often used in catalysis)
O
H
2
N
OH
H
SH
Cysteine (nucleophilic, used in catalysis, controls shape of protein)
O
H
2
N
OH
H
Valine (hydrophobic, bulky)
O
H
2
N
OH
CO
2
H
H
Aspartic acid (acidic, used in catalysis)
NH
O
H
Proline
OH
Amino Acids, Peptides & Proteins
Peptides & proteins:
• Derived from amino acids through peptide or amide bonds.
• The amine and acid ends of amino acids couple to form amide (peptide) bonds in peptides/proteins/enzymes.
• Proteins fold into well-defined structures. The hydrophobic residues segregate to the water-free interior, while the polar/charged residues favor the exterior.
Peptides: Coupling AAs Together
• Peptides & Proteins: Linear oligomers of the 20 amino acids
• Peptides ≤ 20 amino acids; Proteins > 20 amino acids
Functions:
1.
Catalysis - enzymes
2.
Membrane channels
3.
Structural support (boundaries)
4.
Regulate metabolites (storage & transport)
5.
Antibodies; cellular signaling (recognition & binding)
Aspartame
Discovery story:
• In 1965 by Jim Schlatter working on discovering new treatments for gastric ulcers.
• Made a dipeptide intermediate, which he spilled on his hand
• Tested the dipeptide in coffee
O
CH
3
O
H
HN O
Methyl ester
Phenylalanine
H
3
N
H
O
Aspartic acid
O
Aspartame
• 4 calories per gram
• 180 times sweeter than sugar
Aspartame: A Dipeptide
O
CH
3
O
H
HN O
Methyl ester
Phenylalanine
H
3
N
H
O O
Aspartic acid
Two main constituents:
Phenylalanine
Aspartic acid
Goal:
1. Make the methyl ester of phenylalanine
2. Make a peptide (amide) bond between phenylalanine and aspartic acid
Overall - two main steps to this synthesis
Dipeptides: Coupling of 2 AAs
Consider the synthesis of the dipeptide val-ala (valine-alanine):
O CH
3
N-terminus C-terminus
H
2
N OH
N
H
O valine alanine
• Coupling of amino acids is an application of nucleophilic acyl substitution
• Issue of selectivity arises: val + ala
val-ala + ala-val + val-val + ala-ala
A mixture of 4 possible amide products
Merrifield’s Solid-Phase Synthesis
In order to get the desired peptide (val-ala), the appropriate NH
2 units must be joined.
and CO
2
O CH
3
H
2
N
OH H
2
N
OH
O valine's C-terminus alanine's N-terminus
The selectivity is accomplished through the use of protecting groups.
Merrifield’s approach:
1.
Protect N-terminus of valine
2.
Protect C-terminus of alanine
3.
Couple valine and alanine
4.
Deprotect to get dipeptide
Merrifield’s Solid-Phase Synthesis
1. Protection of valine’s N-terminus:
O
O O
Cl
H
2
N
OH
(FMOC-Cl)
O
Fmoc group
O
H
N
O
OH
Merrifield’s Solid-Phase Synthesis
2. Protection of alanine’s C-terminus:
Attach the C-terminus to a plastic bead (solid-phase synthesis!)
CH
3
CH
3
OH O
H
2
N H
2
N
O O
Benefits of solid-phase:
• Ease of attachment
• Ease of removal; just filter away from product solution
Merrifield’s Solid-Phase Synthesis
3. Couple valine and alanine:
N C N
DCC
(Used to derivatize the CO
2
H to make it more reactive)
O
Fmoc group
O
H
N
O
OH
+
H
2
N
CH
3
O
O
Coupling agent such as DCC
(dicyclohexylcarbodiimide)
O
H
N
O
O CH
3
N
H
O
O
Merrifield’s Solid-Phase Synthesis
3. Deprotection of Fmoc & bead:
O
H
N
O
O CH
3
N
H
O
O
1. Piperidine (to remove Fmoc)
2. CF
3
CO
2
H (TFA, to remove bead)
H
2
N valine
O CH
3
N
H
O alanine
OH
Proteins
• Amino acid polymers; when long enough, they fold back on themselves to create intricate, well-defined 3D structures
• The structure of a protein specifies its function.
• The AA sequence specifies its structure.
• The AA chain typically adopts regional sub-structures which sum together to deliver the overall structure of the protein.
Forces/Factors that dictate protein folding:
1.
Planarity of amide bonds
2.
H-bonding
3.
Hydrophobic interactions
4.
Electrostatic Attraction
5.
Disulfide linkages
Proteins
1. Planarity of amide bonds:
O
H
N
R
O R
N
H
O
O
H
N
R
O R
N
H O
Barrier of rotation
~20 kcal/mol
Proteins
2. H-bonding:
O
H
N
O
H
N
R
O
N
H
N
H
O R
R
R
O
O
H-bond worth ~ 5 kcal/mol
H-bonds orient the chain
3. Hydrophobic Interactions:
Lots of hydrophobic interactions between Rs and H
2
O unstable
Proteins
H
N
O
N
H
Ph
H
N
O
O
Protein folds to “clump” R groups together in the interior of protein to avoid
H
2
O - very energetically favored
Fold
O
Hydrophobic pocket
N
H
O
NH
NH
O
4. Electrostatic Attraction:
Proteins
NH
3 O
O
Proteins
5. Disulfide Linkages:
HS
SH
Mild oxidant
S S • Covalent S-S
• Drastically alters shape
• Worth ~ 50 kcal/mol
Proteins
Overall, these 5 structural/energetic features leads to the final 3D protein structure. However, predicting the structure from the amino acid sequence is still a challenge.
Hierarchy of Structural Elements of Proteins
1.
Primary structure: AA sequence
2.
Secondary structure: discrete sub-structural elements (modules) a
-helix & b
-sheet a
-helix: see board for depiction
Note:
1.
Internal H-bonding
2.
The way the side chains line up
3.
3.6 AAs per turn b
-sheet: see board for depiction
Note:
1.
Chain-to-chain H-bonding
2.
Alternating (up-down, up-down)
Pattern of R groups
Proteins
Hierarchy of Structural Elements of Proteins
3. Teritary Structure: the individual secondary structural elements organized in 3D.
See board for depiction.
4. Quaternary Structure: non-covalent complexation of different proteins.
Lipids
• Structurally diverse, derived from living organisms
• Functional theme is hydrophobicity - water avoiding due to long alkyl chains
• Often found at the interface of aqueous compartments
3 Major Classes of Lipids:
1.
Fats and oils
2.
Phospholipids
3.
Cholesterol & derivatives (steroids)
Lipids
1.
Fats & Oils
Derived from glycerol and fatty acids:
H
CH
2
OH
OH
CH
2
OH
Glycerol: a triol (3 nuc sites)
+
Palmitic acid (C
16
fatty acid) couple
Weak intramolecular attractive forces between chains
O
O
O
O
O
O
O
OH
Triacyl glyceride
Lipids
1.
Fats & Oils
• In order for a fat to melt, these weak dispersive forces must be broken.
• More contacts, the better the packing and the higher the m.p. of the fat
• Less contacts, worse packing of chains, the lower the m.p.
Unsaturated Fats: O
O
O
O
O
O No contacts here due to Z-alkene
Oils are polyunsaturated - lots of alkenes & have low mp due to less packing
Butter has very little unsaturated & has higher mp
Soaps & Detergents
• Hydrolyzed fats
• A long chain carboxylate molecule:
Lipids
O
O
-
Na
+
Polar, hydrophilic end
Non-polar, hydrophobic end
Lipids
Soaps & Detergents in H
2
O:
O
O
-
Na
+
O
O
-
Na
+
O O
-
Na
+
H
2
O
In H
2
O, forms a micelle .
+
Na
-
O
O
Hydrophobic
Interior
Grease & dirt get trapped in the interior.
Micelle is H
2
O soluble so can wash out dirt.
H
2
O
+
Na
-
O
O
+
Na
-
O
O
+
Na
-
O
O
+
Na
-
O
O
H
2
O
+
Na
-
O O
+
Na
-
O
O
+
Na
-
O
O
O
O
-
Na
+
O
O
-
Na
+
O
O
-
Na
+
O
O
-
Na
+
O
O
-
Na
+
Hydrophilic
Exterior
H
2
O
Lipids
2. Phospholipids:
• Have hydrophobic and hydrophilic regions
• Forms membranes
• Precursors to prostaglandins
O
O
O
O
O
O P O
O
N
H
2
O insoluble
Phosphatidyl choline
Highly charged
H
2
O soluble
Lipids
2. Phospholipids:
• Forms membranes: self-organize at certain concentrations to form bilayers
• Membranes are largely impermeable to charged species that exist in biological environments.
H
2
O outside of cell
N
N
N
N
O
O
P
O
O
O
O
O
O
O
O
P
O
O
O
O
O
O
O
O
P
O
O
O
O
O
O
O
O
P
O
O
O
O
O
O
Cell membrane
Hydrophobic interior
O
O
O
O
O
O
P
O
O
O
N
O
O
O
O
O
P
O
O
N
O
O
O
O
N
O
O
P
O
O
O
O
O
O
N
O
O
P
O
O
H
2
O inside of cell
3. Cholesterol & Steroids
Cholesterol:
27 carbons
4 rings
8 stereocenters
Derived from terpenes
HO polar
Lipids
H
H H non-polar
Cholesterol is a precursor to several steroidal hormones:
Testosterone (male hormone)
Estrone (female hormone)
Lipids
Cholesterol is a precursor to several steroidal hormones:
Testosterone (male hormone)
Estrone (female hormone)
OH
H
H H
HO
O
Testosterone
H
H
Estrone
H
O
These hormones operate at the genetic level (turn genes on and off) to control biochemistry. They are recognized by specific protein receptors.
Antioxidants & Chocolate
Antioxidants:
• Protect against cardiovascular disease, cancer and cataracts
• Thought to slow the effects of aging
QuickTime™ and a
TIFF (LZW) decompressor are needed to see this picture.
Chocolate:
• High levels of antioxidants - complex mixtures of phenolic comounds
• By weight, has higher concentration of antioxidants than red wine or
Green tea
• 20x higher concentration of antioxidants than tomatoes
QuickTime™ and a
TIFF (LZW) decompressor are needed to see this picture.
Dark chocolate has more than 2x the level of antioxidants as milk chocolate.
Side note: The main fatty acid in chocolate, stearic acid, does not appear to raise blood cholesterol levels the way other saturated fatty acids do.