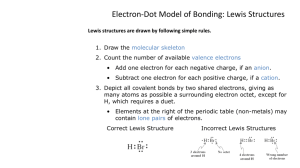
the procedure in writing Lewis structures
advertisement

Principles of drug Synthesis . Some common structures Positions of substituents Positions of aromatic substituents Names and abbreviations for carbon chains Various alcohol types Alkene, alkyne, alcohol, ether Amine , nitro How to draw Lewis’ structure correctly Aldehyde, ketone, acid, ester amide oxidation levels Various oxidation levels of carbon atom A review of bonding theory the ionic bonding force arises from the electrostatic attraction between ions of opposite charge the covalent bonding force arises from sharing of electron pairs between atoms. The Lewis structure notation essential qualitative information about properties of chemical compounds. chemical properties of the groups that make up organic molecules are to a first approximation constant from molecule to molecule an atom and its valence electrons The element symbol represents the core (the nucleus and all the inner-shell electrons) The core carries a number of positive charges + charge = the number of valence electrons The electrons are shown explicitly Ions are obtained by adding or removing electrons The charge on an ion = core charge - number of electrons shown explicitly A covalent bond model is constructed by allowing atoms to share pairs of electrons. Ordinarily, a shared pair is designated by a line: C-C H-H . All valence electrons of all atoms in the structure must be shown explicitly. Those electrons not in shared covalent bonds are indicated as dots For ion containing > 2 atoms covalently bonded to each other the total charge on the ion = the total core charge - the total number of electrons (shared and unshared) Lewis structures all unshared electrons around the atom and all electrons in bonds leading to the atom must be counted. The valence-shell occupancy must not exceed 2 for hydrogen and must not exceed 8 for atoms of the first row of the periodic table. For elements of the second and later rows, the valence-shell occupancy may exceed 8. Formal charge - a bookkeeping device for electrons - a rough guide to the charge distribution within a molecule Assign to each atom all of its unshared pair electrons and half of all electrons in bonds leading to it its electron ownership. The formal charge of each atom = core charge – electron ownership The electron ownership of H is 1, its core charge is + 1 its formal charge = zero The electron ownership of oxygen is 7, and the core charge is +6 formal charge = -1 . All nonzero formal charges must be shown explicitly in the structure. the procedure in writing Lewis structures 1. 2. 3. 4. 5. Count the total nb of valence electrons contributed by the electrically neutral atoms. for ion add one e to the total for each negative charge; subtract one for each positive charge. Write the core symbols for the atoms and fill in the number of electrons determined in Step 1. The electrons should be added so as to make the valence- shell occupancy of hydrogen 2 and the valence-shell occupancy of other atoms not less than 8 wherever possible. Valence-shell occupancy must not exceed 2 for H and 8 for a firstrow atom; for a second-row atom it may be 10 or 12. Maximize the number of bonds, and minimize the number of unpaired electrons, always taking care not to violate Rule 3. Find the formal charge on each atom. Lewis structure of NO2 a class of structures, for which the properties are not those expected from the Lewis structure. The thermochemical properties of various types of bonds are in most instances transferable with good accuracy from molecule to molecule the heat of hydrogenation of benzene is less exothermic by about 37 kcal mole-1 than one would have expected from Lewis structure on the basis of the measured heat of hydrogenation of ethylene a discrepancy of this magnitude requires a fundamental modification of the bonding model. . 1 2 another Lewis structure of benzene, 2, is identical to 1 except for the placement of the double bonds. The superposition of two or more Lewis structures into a composite picture is called resonance the term resonance tends to convey the idea of a changing back and forth with time incorrect idea difficult to avoid the pitfall of thinking of the benzene molecule as a structure with three conventional double bonds, of the ethylene type, jumping rapidly back and forth from one location to another The electrons in the molecule move in a field of force created by the six carbon and six hydrogen nuclei arranged around a regular hexagon Each of the six sides of the hexagon is entirely equivalent to each other side; there is no reason why electrons should, even momentarily, seek out three sides and make them different from the other three, as the two alternative 1 and 2 seem to imply that they do. A less misleading picture: the circle in the middle of the ring implies a distribution of the six double bond electrons of the same symmetry as the arrangement of nuclei. 1 2 We shall continue to use the notation 1 and 2, as it has certain advantages for thinking about reactions. The most important features of structures for which resonance is needed: the molecule is more stable (of lower energy) than one would expect from looking at one of the individual structures, the actual distribution of electrons in the molecule is different from what one could expect on the basis of one of the structures. resonance is often referred to as delocalization. electrons are free to move over a large area of the molecule Many other structures are of the necessity for modifying the Lewis structure language by the addition of the resonance concept The carboxylic acids are much stronger acids than the alcohols due largely to greater stability of the carboxylate ion (6) over the alkoxide (7 ) . The allylic system may be cation (8), anion (9), and radical (10), are all more stable than their saturated counterparts. there is for each an alternative structure The rules in using resonance notation 1. All nuclei must be in the same location in every structure. 2. Structures with nuclei in different locations, for example 15 and 16, are chemically distinct substances, and interconversions between them are actual chemical changes, always designated by 3. Structures with fewer bonds or with greater separation of formal charge are less stable than those with more bonds or less less charge separation. Thus 11 and 12 are higer-energy, respectively, than 13 and 14. 1. Molecular Geometry Lewis structures provide a simple method of estimating molecular shapes. The geometry about any atom covalently bonded to two or more other atoms is found by counting the number of electron groups around the atom. Each unshared pair counts as one group, and each bond, whether single or multiple, counts as one group. The number of electron groups around an atom is therefore equal to the sum of the number of electron pairs on the atom and the number of other atoms bonded to it. . The geometry linear if the number of electron goups is two trigonal if the number is three, tetrahedral if the number is four . The rule is based on the electron-pair repulsion model electron pairs repel each other, they will try to stay as far apart as possible. if the electron groups are all equivalent the shape will be exactly trigonal (120o bond angles), or exactly tetrahedral (109.5o bond angles), BH3 or CH3+ (trigonal), CH4 or NH4+ (tetrahedral). . If the groups are not all equivalent, the angles will deviate from the ideal values. Thus in NH3 (four electron-groups, three in N-H bonds, one an unshared pair), the unshared pair, being attracted only by the nitrogen nucleus, will be closer to the nitrogen on the average than will the bonding pairs, which are also attracted by a hydrogen nucleus. Therefore the repulsion between the unshared pair and a bonding pair is greater than between two bonding pairs, and the bonding pairs will be pushed closer to each other. The H-N-H angle should therefore be less than 109.5o (It is found experimentally to be 107o) in H2O (four electron groups, two unshared pairs, and two O-H bonds), the angle is 104.5o.