Lectures 8-10
advertisement

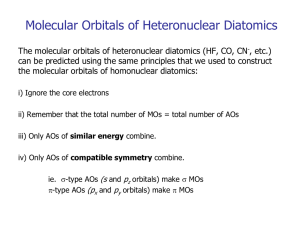
Triatomics and Beyond 1) Complex, so we deal with simple symmetrical molecules 2) Same principles apply to orbital combinations as with Diatomics: i) ii) Compatible symmetry Compatible energy (within 1 Rydberg, 1 Ry) 3) The number of valence AO’s must equal the number of Mo’s 4) MO’s must conform to the symmetry of the molecule. 5) Orbitals of the same energy and the same number of nodes mix. BeH2 Be BeH2 is the simplest triatomic molecule. 2pz Linear the gas phase. 2pxy 2p The relative energies for the AO’s of Be and H are: 2s2s 1s 1s Energy 1s (Be) = -9.38 Ry 1s (H) = -0.99 Ry 2s (Be) = -0.61 Ry 2p (Be) = +0.14 Ry 1s 1s Be Along bond axis Which atomic orbitals will combine to make σ MOs? Which will combine to make H p MOs? Which will not combine remaining σ or p nonbonding MOs? BeH2 H (x2) BeH2 MO Diagram 4s* Along bond axis HH*x 22 Be Be 3s* 2pz 2pxy 2p 1p 1p 2s Energy 1s 2s Lewis Structure? Electron Configuration? BO? HOMO? LUMO? Lewis Acid? 1s CO2 Lewis Structure? Shape Family? Valence atomic orbitals on C and O: 2s and 3 x 2p Consider s and p MO’s formed separately. 6 s and 6 p MO’s will be formed (12 possile for each) C 9s O*2 8s 2pz Order of energies: Energy 2s (O) + 2s(C) small 2s (O) + 2pz(C) smallest 2pz(O) + 2s(C) large 2pz(O) + 2pz(C) largest 3p 2p 2pxy 2pz 2p 2pxy 2p 2s 1p 7s 2s 6s Along bond axis Along bond axis 5s 4s Valence MO Diagram for CO2 2s (O) + 2s(C) small 1s, 2s, 3s*, 2s 6s* 2s (O) + 2pz(C) smallest 3s, 2s, 3s, 4s* 5s* 2pz(O) + 2s(C) large 4s, 5s*, 4s, 3s 9s 8s 3p 2p 2pz(O) + 2pz(C) largest 6s*, 5s, 4s, 5s, 2px(O) + 2px(C) largest 1p , 2p, 3p*, 2p 2py(O) + 2py(C) largest 1p , 2p, 3p*, 2p Energy 2p 2p 2s 4s Free atom 3s* 2s 1s C 1p 7s 6s 5s 4s CO2 2s Free atom O (x2) BH3 Lewis structure? Shape Family? B HH x*3 3 B 2p 2pxy 2pz 2s Along Bonding Plane What orbital combinations are possible now? 1s BH3 MO Diagram 4s* HH x*3 3 B B 3s* 2p 2pxy 2pz 3s* 1p Energy 2s 1s Along Bonding Plane 2s 2s 1s CH4 - The third dimension… Frontier MO Theory BH3 Reactions take place during collisions. 4s* Hx3 B 3s* Bonds are formed and/or broken. 2p That must mean that there is Energy some kind of orbital interaction. Which orbitals are most likely interact in forming the new bond? H- 3s* 1p 2s 2s Free atom 1s 2s 2s 1s Free atom 1s BH3 + H- —> BH4In general, reactions take place via the interaction of the HOMO of one component with the LUMO of the other because these are the closest in energy. These orbitals are known as the “frontier orbitals”. Electron delocalization (Resonance) In resonance structures, the only electrons that move are: O O O O O Delocalized electrons are always found in O p orbitals. As p orbitals are usually found at higher energy than the s orbitals, the HOMO and LUMO of molecules with multiple bonds are usually As a result of this, we often look only at the diagrams. p orbitals. p orbitals and construct p MO Ethylene H H C 2p* C H H C: 2*(2s + 3*(2p)) => 8 AO’s H: 4*(1s ) => 4 AO’s => 12 AO’s s*’s 1p 5s 4s 3s 2s => 12 MO’s 1s p-MO diagram of Ethylene H Ozone H C H C H O O O 2p* O O O 3p* C2p C2p O2p 2pnb O2p 1p Nodes… Pi-bond order… Sigma bond order Total bond order = p bond order + s bond order When Ethylene reacts… Ethyne? 1p Nodes… Sigma & Pi-bond order… Total bond order Lewis BO Formal Charge: Butadiene Ethylene H H C C H H H H LUMO C C 2p* 1.2 H C C H H H 4p* 2 0.2 -7.3eV C2p 12.2 ev C2p 9.7ev C2p -11 3p* LUMO 1p HOMO -9.5 HOMO 2p -12 Nodes… The importance of the HOMO/LUMO gap. C2p 1p Note: this is not two isolated double bonds but a single p-system spread out over four carbons. Benzene H H H H C H H C H C C C C C C C C C H H H C H H The polygon method for determining p-MOs of monocyclic unsaturated molecules: 4p* 3p* 3p* 2p 2p 1p Works for any monocyclic molecule with contiguous atomic p orbitals. The p-MOs of Benzene How many pi-electrons? Nodes…(Cuts?) Aromatic Stabilization (1,3,5-hexatriene) 3 4p* 3p* 3p* 2p 2p 2 1 1p Benzene can’t be considered to have “three double bonds and three single bonds”. It has three p bonds with bond order _____. Accordingly, all six C-C bonds in benzene are 140 pm (whereas pure C-C bonds are 154 pm and pure C=C bonds are 134 pm). 0