Biology
advertisement

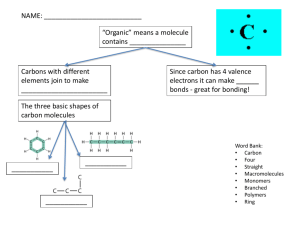
Biology Señor Robles 70 Things I know about Macromolecules I am soooooooo smart Carbohydrates 1. What is the general term used for macromolecules containing only the elements carbon, hydrogen, and oxygen? 2. What molecular formula do (almost) all monosaccharides share? 3. What do you call molecules (such as the monosaccharides glucose and fructose) which share the same formula but are arranged differently? 4. What chemical indicator is used to show the presence of monosaccharides? 5. When two monosaccharides are chemically bonded together, what is the general term for the resultant (and twice as large) molecule? Hint: read the next two questions. 6. A water molecule is removed in the formation of a bond linking together monosaccharides. What are the two names for this process? 7. A disaccharide can be broken into its component parts by the addition of water. What’s this process called? 8. Glucose + Glucose → ? 9. Glucose + Fructose → ? 10. What do you call any chain of glucose molecules linked together? 11. What type of atom connects any two glucose molecules in any polysaccharide (or any two monosaccharides in a disaccharide)? 12. Glucose + Glucose + Glucose + Glucose + Glucose etc. in long chains side by side → ? 13. What type of bond holds these chains together on their sides? 14. Where is the above polysaccharide found, and what is it used for? 15. What do we call it in the human diet, and how is it beneficial? 1 16. What is the storage form of sugar found in the roots and tubers of plants? Describe its shape. 17. What is the principle storage form of sugar found in vertebrates? (Your liver makes and stores this when there is too much glucose in your blood.) How do you describe its shape? 18. What modified polysaccharide can be found in animals, in the form of the tough exoskeleton of insects and crustaceans? 19. Where else can this structural material be found? 20. How is this polysaccharide “modified?” 21. What are the monomers that link together to form any carbohydrate polymer? 22. What is the difference between the terms polymer and polysaccharide? 23. What chemical indicator tells of the presence of polysaccharides like starch? 24. What simple sugar seems to be the sweetener of McDonald’s shakes? 25. Describe how it is made: 26. What is the main health concern with HFCS? 27. How is this reflected in the human population? 28. How is it that fructose is more fattening than sucrose? 29. Draw the structural formula for glucose. Lipids 30. What lipids (or fats) are structurally different from other lipids because they are composed of four linked carbon rings? 31. What are three examples of these special lipids? 32. What lipids are composed of two fatty acid chains linked to a glycerol and a phosphate group? 2 33. Lipids don’t like water. What term describes this? 34. What term describes the region of some fat molecules that is water tolerant? 35. Most fats/lipids are involve with energy storage, but some (like phospholipids and wax) have other purposes. What are they involved with? 36. If you were stranded on a desert island with very little food, would you rather have a pound of starch or a pound of lard? Why? 37. Fatty acids (and amino acids) always have this trademark group of four atoms: 38. What does an unsaturated fat molecule have that a saturated fat molecule does not? 39. When your body burns fat, and therefore oxidizes fatty acids, what poisonous compound is released into your blood? 40. What term applies to the fat molecule made of a single glycerol molecule attached to three fatty acid chains? 41. What is the difference between HDLs and LDLs? 42. What are trans fats, and what’s wrong with them? 43. Why do fattening foods taste so good? Proteins 44. What element does protein always contain in addition to the three elements found in carbohydrates and lipids? 45. What other two elements are found in select proteins (think cysteine and hemoglobin)? 46. What monomers link up to form proteins? 47. What special proteins have names that end (usually) with the suffix --ase? 48. Circle and identify the component parts of an amino acid (make sure to explain the significance of the R): 3 49. What term applies to enzymes because they enable reactions to occur more quickly? 50. A protein is composed of a string of amino acids linked together. What do you call this string? 51. What kind of bond holds these amino acids together? How is it formed? 52. What do you call the four levels of organization of protein molecules? 53. What are two examples of the secondary structure of protein? 54. Fibrous protein includes collagen, elastin, silk, and keratin. Which one of these is abundant in skin, claws, hair, feathers, and hooves? 55. What protein is involved in the transport of oxygen in your bloodstream? 56. What liquid indicator changes color when heated in the presence of protein? 57. What are two membrane proteins found suspended in cell membranes? What do these two proteins do? 58. What class of proteins transmits messages throughout your body? 59. Describe the role of insulin, and the cause of type 2 Diabetes: Nucleic Acids 60. What are the monomers of nucleic acids? 61. What are the two principal types of nucleic acids, and where do each reside? 62. What are the three components of a nucleotide? (label the diagram) 4 63. Which particular sugar is part of the DNA molecule? 64. This sugar is described as a pentose sugar. What does that mean, and why is it specified? 65. What are the four names of the nitrogen bases which comprise the “rungs of the ladder” of the DNA molecule? 66. How do the nitrogen bases pair up? 67. Besides carbon, hydrogen, and oxygen, what other two elements are always found in nucleic acids? 68. Label this little section of DNA: G A 69. Once this nucleic acid gets long enough, what characteristic and famous shape does it have? 70. How many base pairs does your (as a species) particular genome have? 5