C5. Electricity and chemistry - IGCSECoordinatedScience-Dnl
advertisement

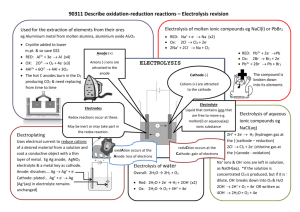
Conduction of electricity conductor is a solid which allows electricity to pass through but is not chemically changed during the conduction presence of freely moving valence electrons e.g. all metals and graphite conducting rods & wires are used in electrolysis cells non-conductor is a solid which does not allow electricity to pass through, a.k.a insulator valence electrons are held in fixed positions e.g.. sulfur, phosphorus, diamond, solid state crystalline salts, wood and glass etc. Electrolysis Cell (Source of electricity) electrolysis is the process of breaking up an Conducting wire Beaker Cathode Electrode Anode Electrode Electrolyte ionic compound into simpler substances, usually elements, using electricity electrolysis occurs when the ionic compound is in aqueous solution or molten because it allows electric current to pass through electrolysis is carried out in an electrolysis cell electricity is passed from a cell or battery through a liquid which may be a aqueous solution or molten solid a molten ionic compounds or aqueous solution of ionic compounds that allows electricity to pass through is called an electrolyte the rods which carry the electric current to and from the electrolyte are called electrodes Electrodes electrodes are rods that carry the electric current to and from an electrolyte they are usually; platinum, copper, graphite Cathode: electrode that is connected to the negative terminal of the cell or battery. positively charged ions, cations, moved towards the cathode Anode: electrode that is connected to the positive terminal of the cell or battery. negatively charged ions, anions, moved towards the anode Electrolytes and non-Electrolytes electrolytes: molten ionic compounds or aqueous solution of ionic compounds that allows electricity to pass through and are decomposed in the process e.g.. acids, alkali, salts dissolved in water, molten salts non-electrolytes: does not allow passage of electricity e.g.. distilled water, alcohol, turpentine, oil, paraffin, organic solvents Electrolysis when electricity is passed through an electrolyte, chemical decomposition occurs this involves the ‘splitting up’ of the electrolyte since all electrolytes are ionic, composed of positively charged ions (cations) & negatively charged ions (anions) when an electric current pass through the electrolyte, ions in the solution or liquid migrate towards the oppositely charged electrode discharge of ions at the electrodes results in the chemical decomposition of the electrolyte to form its elements at the anode, negatively charged ions lose their electron(s) to the anode to form neutral atoms the negatively charged ions are said to be oxidized and discharged at the anode oxidation occurs at the anode at the cathode, positively charged ions gain electron(s) from the cathode to form neutral atoms the positively charged ions are said to be reduced and discharged at the cathode reduction occurs at the cathode metals or hydrogen are formed at the negative electrode (cathode), and that non-metals (other than hydrogen) are formed at the positive electrode (anode). Electrolysis of molten Compounds many ionic compounds are binary compounds i.e. compounds containing only 2 elements e.g. KI, PCl2, NaCl binary ionic compound contains a metal cation and a non-metal anion electrolysis of a molten binary compound will yield a metal and a non-metal as products metal will be formed at the cathode while non-metal will be formed at the anode Electrolysis of molten Compounds Product formed at; Compound Electrolysed Cathode (-) Anode(+) Copper Bromide Copper Bromine Zinc Chloride Zinc Chlorine Sodium Chloride Sodium Chlorine Aluminium Oxide Aluminium Oxygen Electrolysis of Molten Lead(II) Bromide Electrolysis of Molten Lead(II) Bromide At the cathode: Pb2+ ions gain electrons from the electrodes to become lead atoms Pb2+ ions have been discharged and molten greyish globules of lead metal are formed below the electrolyte electrode reaction at the cathode; Pb2+(l) + 2e→ Pb(l) At the anode: Br- ions lose electrons to electrode to become bromine molecules - Br- are oxidized Bromine atoms combine to form bromine molecules forming an effervescence of pungent, red-brown bromine gas electrode reaction at the anode; 2 Br- (l) → Br2(g) + 2e- Predicting products in the electrolysis of aqueous solutions using inert electrodes e.g. graphite; Electrolysis of Aqueous Solutions an aqueous solution of an ionic compound is a mixture of 2 electrolytes – one of which is water although water is molecular, in aqueous solutions, a small % of it ionizes into hydrogen ions & hydroxide ions; H2O(l) → H+(aq) + OH-(aq) e.g. aqueous copper (II) sulphate; contains water & copper (II) sulphate water ionises into; hydrogen ions & hydroxide ions copper (II) sulphate ionises into; copper (II) ions and sulphate ions ions discharged at the electrodes depends on the position of the ions in the electrochemical series Rules for Predicting Selective Discharge of Cations cations from the metals lowest in the reactivity series are discharged at the cathode in preference to any other cation present in the solution cations of less reactive metals e.g. Cu2+, Au+, Ag+ are preferentially discharged otherwise, H+ ions from water will be discharged to form H2 gas 2H+(aq) + 2e- H2(g) cations of very reactive metals (Na+, K+, Ca2+) cannot be discharged in the presence of water, so they remain in solution Rules for Predicting Selective Discharge of Anions OH- ions from water are preferentially discharged when the solutions are dilute, to form O2 4OH-(aq) → O2(g) + 2H2O(l) + 4e anions such as Cl-, Br- and I- can be preferentially discharged when their concentrations are high enough when compared to OH when SO42- and NO3- are present in water, it is the OH- from water which is preferentially discharged Product formed at; Electrolyte Cations & anions present Cathode (-) Anode(+) Concentrated sodium chloride Na+, H+, Cl- & OH- Hydrogen Chlorine Dilute sulfuric acid H+, SO42- & OH- Hydrogen Oxygen Dilute sodium chloride Na+, H+, Cl- & OH- Hydrogen Oxygen Concentrated hydrochloric acid H+, Cl- & OH- Hydrogen Chlorine Dilute Silver nitrate Ag+, H+, NO3- & OH- Silver Oxygen Dilute Copper nitrate Cu2+, H+, NO3- & OH- Copper Oxygen Dilute hydrochloric acid H+, Cl- & OH- Hydrogen Oxygen Electrolysis of dilute sodium chloride solution at the anode; anions OH- & Cl- moves to the anode OH- ions are selectively discharge according to the position of ions in the electrochemical series gas bubbles are formed which re-lights a glowing wooden splinter -this gas is oxygen 4OH-(aq) 2H2O(l) + O2(g) + 4e at the cathode; cations H+ & Na+ moves to the cathode H+ ions are selectively discharge according to the position of ions in the electrochemical series gas bubbles are formed, when a lighted wooden splinter is placed near the test tube, a 'pop' sound is produced – this gas is hydrogen gas 2 H+(aq) + 2e- H2(g) Electrolysis of dilute sulfuric acid Electrolysis of dilute sulphuric acid dilute sulphuric acid can be electrolysed using the apparatus shown at the cathode: H+ ions attracted to the cathode electrode reaction at the cathode(-); 2H+(aq) + 2e- → H2(g) hydrogen gas is produced at the cathode (gas B) at the anode: both OH- and SO42- will be attracted to the anode but OH- ions are preferentially discharged electrode reaction at the anode(+); 4 OH-(aq) → O2(g) + 2H2O(l) +4e oxygen gas is produced at the anode (gas A) overall Reaction; 2H2O(l) → 2H2(g) + O2(g) this reaction is sometimes known as electrolysis of acidified water sulphuric acid is added to increase the number of mobile ions to help conduct electricity in this process, the amount of acid remains the same, but amount of water decreases - hence, the concentration of sulphuric acid increases how do you test for gas A (oxygen) & gas B (hydrogen)? Electrolysis of aqueous copper(II)sulphate using carbon electrodes copper (II) sulfate solution contains the following ions; blue Cu2+, SO42- and H+ & OH- from water Cu2+ & H+ migrate to the cathode while SO42- & OH- migrate to the anode at the cathode; Cu2+ ions are preferentially discharged and deposited on the cathode as a brown deposit of solid copper Cu2+(aq) + 2e → Cu(s) at the anode; OH- ions are preferentially discharged and oxygen bubbles are given off 4 OH-(aq) → O2(g) + H2O(l) +4e- the blue colour of copper (II) sulfate solution fades as Cu2+ ions are discharged Electrolysis of aqueous copper(II)sulphate using copper electrodes copper (II) sulfate solution contains the following ions; blue Cu2+, SO42- and H+ & OH- from water Cu2+ & H+ migrate to the cathode while SO42- & OHmigrate to the anode at the cathode; Cu2+ ions are preferentially discharged and deposited on the cathode as a brown deposit of solid copper thus the cathode grows thicker Cu2+(aq) + 2e- → Cu(s) at the anode; electrodes are not inert, copper loses electrons forming copper ions which goes into solution Cu(s) → Cu2+(aq) + 2e the anode gets thinner Cu2+ ions formed at the anode move to the cathode where they are deposited the blue colour of copper (II) sulfate solution does not fade because Cu2+ ions removed from the solution are replaced by those from the anode Electroplating electroplating is the application of a thin layer of metal to a metallic or other conducting surface through electrolysis the object to be plated is made the cathode (- ve electrode) of an electrolysis cell the anode is usually a bar of the plating metal the object is immersed in an aqueous solution containing cation of the plating metal during electrolysis, the plating metal (anode) dissolves forming cation which are deposited on the object (cathode) as metal atoms Electroplating steel key with copper to which terminal of the battery is the copper foil & steel key connected to? copper foil is connected to the + ve terminal to become the anode & while the steel key is connected to the - ve terminal to become the cathode suggest a suitable substance to be used as an electrolyte aqueous solution of copper (II) sulfate/nitrate/chloride what observations would be made at the electrodes when electric current is passed through the electrolyte for some time? anode;- copper foil gradually become thinner Cu(s) → Cu2+(aq) + 2e cathode;- steel key gradually get coated with copper Cu2+(aq) + 2e→ Cu(s) giving a reason, predict what observation(s) will be made in the electrolyte during electrolysis the colour of the copper sulfate electrolyte remains the same as the Cu2+ ions removed from the solution at the cathode are replaced by Cu2+ ions formed at the anode Uses of Electroplating nickel, silver, tin & gold are the most commonly used metals for electroplating articles are electroplated to; improve their appearance;- plating gives the object a shiny surface that does not get dull, - gold plating is used for jewelry to provide a protective coating;steel objects are coated with a layer of tin which protects the metal underneath from air and water thus protecting it from rusting to give them special surface properties to give them engineering or mechanical properties Manufacture of Cl2, H2 & NaOH from brine brine is a concentrated aqueous solution of sodium chloride i.e. sodium chloride (NaCl) dissolved in water (H2O) brine is obtained from sea water or from rock salt underground electrolysis of brine is used to manufacture chlorine, sodium hydroxide & hydrogen on a large scale chlorine is used for making disinfectants & bleaches, manufacture of hydrochloric acid and for water treatment sodium hydroxide is used for making soap & in extraction of aluminium hydrogen is used for manufacture of ammonia, making margarine & as a pollution-free fuel Electrolysing brine brine is electrolyzed in a diaphragm cell shown in the diagram aqueous sodium chloride contains; Na+, H+, Cl- & OH- ions Na+& H+ migrates to the cathode while Cl- & OH- migrates to the anode at the anode; Cl- ions are preferentially discharged forming chlorine gas 2Cl-(aq) → Cl2(g) + 2e- at the cathode; H+ ions are preferentially discharged forming hydrogen gas 2H+(aq) + 2e- → H2(g) Na+ & OH- ions remain in the solution forming an aqueous sodium hydroxide (NaOH) solution Manufacture of aluminium from aluminium oxide extraction of aluminium from aluminium aluminium is the most abundant metal in Earth’s crust – it occurs as metal ore called bauxite which contains about 50% Al2O3 bauxite is purified to yield a white powder, aluminium oxide, from which aluminium can be extracted oxide is done by electrolysis first the aluminium oxide must be molten to allow electricity can pass through it aluminium oxide has a very high melting point (2,070°C), so it would be expensive to melt it instead, aluminium oxide is dissolved in molten cryolite, sodium aluminium fluoride (Na3AlF6), which has a lower melting point (about 1000°C) use of cryolite reduces some of the energy costs involved in extracting aluminium and also improves electrical conductivity electrolysis is carried out using inert graphite electrodes in steel electrolysis cell the electrolyte is molten aluminium oxide Al2O3(s) → 2Al3+(l) + 3O2-(l) at the cathode; aluminium ions (Al3+ )gain electrons forming aluminium metal Al3+(l) + 3e- → Al(l) molten aluminium is siphoned out of the steel electrolysis cell at the anode; oxide ions (O2-) lose electrons forming oxygen atoms which combine to form oxygen gas 2O2-(l) → O2(g) + 4e oxygen reacts with hot carbon anodes forming carbon dioxide thus the anodes needs to be replaced from time to time overall equation for the electrolysis; 2Al2O3(s) → 4Al(l) + 3O2(g) Summary Factors affecting discharge of Ions Uses of electrolysis relative positions of the ions in the purification of metals electrochemical series concentration of the ions in the electrolyte nature of the electrode – inert or reactive electroplating extraction of metals manufacture chlorine, sodium hydroxide & hydrogen from brine Electricity and chemistry – Revision End of Topic C 5 Read your textbook pages 70 to 81 and answer the questions on page 84 & 85 Conductors and insulators (pp82 – 83) is not part of your syllabus but it wont harm your brain if you read through! Best regards ~ Dnl