Hydrocarbon Names and Structures
advertisement

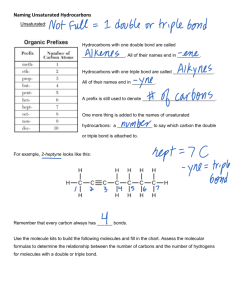
Hydrocarbons & Energy Also Known As… The Stuff That Burns Real Fast & Easy What is a “hydrocarbon”? Hydrocarbons are organic molecules that only contain carbon and hydrogen atoms. Organic molecules are made of carbon (and usually hydrogen), they have energy, and are associated with living things. Carbon is the key element in all organic compounds – especially the hydrocarbons. It forms the skeleton for all hydrocarbons – look at butane below – the carbons are the blue balls in the diagram that make up the “spine” of the structure. Types of Hydrocarbons 1. 2. 3. Hydrocarbons are also known as aliphatic compounds – organic compounds that can be straight, linear chains or cyclical rings. There are three main categories of hydrocarbons within these linear and cyclic molecules… Alkanes – hydrocarbons that have only single bonds within the carbon chain. Alkenes – hydrocarbons that have at least one double bond within the carbon chain. Alkynes – hydrocarbons that have at least one triple bond within the carbon chain. An easy way to remember this is the “A-E-Y” rule – they go alphabetically and numerically with respect to the bonds found between the carbons. Naming Hydrocarbons Hydrocarbons are just like us – they have a first and last name. These names are great because they actually describe the structure of the molecule. The first name indicates the number of carbons in the chain of carbons that makes up the hydrocarbon. This has to be the longest carbon chain possible within the structure. The second name tells you the category of hydrocarbon – alkane, alkene or alkyne? First Names of Hydrocarbons The first name (or “prefix” if you are an English teacher) tells you how many carbons are in the longest chain. Meth = 1 Hex = 6 Eth = 2 Hept = 7 Prop = 3 Oct = 8 But = 4 Non = 9 Pent = 5 Dec = 10 This means that propane will have 3 carbons in its structure and that heptane will have 7 carbons in its structure. The first four are tough…I used to think…”ME & ETHyl went in a PROP plane and it was BUTiful”. That’s awesome – I came up with that all by myself too - I must be some sort of Jenius!!! If you just picked on me for spelling genius incorrectly – you are the real jenius for missing the obvious sarcasm…nice job Einstein! The Last Name The last name (or “suffix”) tells you the type of hydrocarbon you have. If the name ends in… ANE – it is an alkane – all single bonds between the carbon. ENE – it is an alkene – at least one double bond in the carbon chain. YNE – it is an alkyne – at least one triple bond in the carbon chain. This means that pentane, pentene and pentyne all have five carbons but pentane is all single bonds, pentene has at least one double bond and pentyne has at least one triple bond in the carbon chain somewhere…But where??? The Numbers Game Some hydrocarbons will have numbers in their name. These numbers tell you the position of special bond types or side groups that can be found on or within the larger carbon chain. Please note – chemists are LAZY!!! They will always count from the end of the chain that will give them the lowest possible number combinations. You can start counting carbons from either end of the carbon chain to make it easier for you. This means that the carbon that starts off a double or triple bond, or a functional group, will be numbered to tell its position along the chain. Lazy! Lazy! Lazy! First the “e” on the end of mole and then this…What will they do next?!?!?! Allow Myself To Introduce…Myself… Now…lets use all of the names and numbers together… Name the following hydrocarbons! THE END