Ch. 4

Lecture PowerPoint
Chapter 4
Astronomy Today,
5 th edition
Chaisson
Last Revised:
8-Feb-09
McMillan
© 2005 Pearson Prentice Hall
This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning.
Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permitted. The work and materials from it should never be made available to students except by instructors using the accompanying text in their classes. All recipients of this work are expected to abide by these restrictions and to honor the intended pedagogical purposes and the needs of other instructors who rely on these materials.
Chapter 4
Spectroscopy
Solar Spectrum
Na D Lines
Units of Chapter 4
Spectral Lines
The Formation of Spectral Lines
The Energy Levels of the Hydrogen Atom
The Photoelectric Effect
Molecules
Spectral-Line Analysis
Why Do We Care About Spectra?
Almost everything that we study has a spectrum we can use to analyze its composition, motions and many other properties
Wollaston (1802)
First Solar Spectrum - 8 lines seen
Fraunhofer
Fraunhofer (1817)
Fraunhofer lines – A, B, C, D, etc.
Over 500 lines seen
Modern Solar Spectrum
Over one million lines seen!
Fra ünhofer Solar Line Chart
Identification
Table
Ca II H
β
Fe Na I H
α
T a b l e
Original Fra ünhofer spectrum
4.1 Spectral Lines
Spectroscope: splits light into its component colors
Continuous spectrum
Emits BB radiation
Blackbody Curve Spectrum
All colors ( λ’s) emitted
4.1 Spectral Lines
Continuous Spectrum: all colors ( λ’s) present
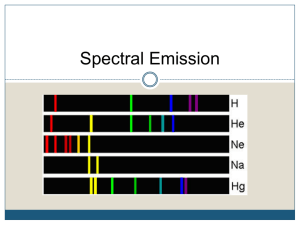
Emission lines : single frequencies emitted by particular atoms
H
Na
He
Ne
Hg
4.1 Spectral Lines
Emission spectrum can be used to identify elements by wavelengths of lines:
600 500 400 nm
4.1 Spectral Lines
Absorption spectrum: if a continuous spectrum passes through a cool gas, atoms of the gas will absorb the same frequencies they emit
Dark line spectra
Continuous spectrum
4.1 Spectral Lines
An absorption spectrum can also be used to identify elements. These are the emission and absorption spectra of sodium (D lines): emission absorption
4.1 Spectral Lines
Kirchhoff ’s laws (the 3 types of spectra):
• Luminous solid, liquid, or dense gas produces a continuous spectrum
• Low-density hot gas produces an emission spectrum
• Continuous spectrum incident on cool, thin gas produces an absorption spectrum
4.1 Spectral Lines
Kirchhoff’s laws illustrated:
4.2 The Formation of Spectral Lines
Existence of spectral lines required a new model of the atom , so that only certain amounts of energy could be emitted or absorbed.
(Planck: ∆E = nhν)
Bohr model had certain allowed orbits for the electron:
(n=1) (n>1)
4.2 The Formation of Spectral Lines
Emission energies correspond to energy differences between allowed levels.
Modern model has electron “cloud” rather than orbit:
4.2 The Formation of Spectral Lines
Absorption can boost an electron to the second (or higher) excited state
Two ways to decay:
1. to ground state
2.
cascade one orbital (energy level) at a time
This second method of energy decay or relaxation can lead to the processes of fluorescence, phosphorescence and laser action
4.2 The Formation of Spectral Lines
Energy levels of the hydrogen atom, showing two series of emission lines:
Note:
Balmer series transitions all start or end at n = 2 level visible
Lyman series (n = 1) ultraviolet
Paschen series (n = 3)
Brackett series (n=4)
Pfund series (n=5) all in infrared
How Transitions Make Spectral Lines
Transitions between energy levels of system
Up: absorption
Down: emission
H
β
Emission
H
Absorption
Energy (eV) Levels of H-atom
UV VIS IR
4.2 The Formation of Spectral Lines
Absorption spectrum : created when atoms absorb photons of right energy for excitation
Multielectron atoms : much more complicated spectra, many more possible states
Ionization changes energy levels
4.2 The Formation of Spectral Lines
Emission nebula
(a) direct decay
(b) cascade
4.2 The Formation of Spectral Lines
Emission lines can be used to identify atoms in nebulae, stars and galaxies:
4.2 The Formation of Spectral Lines
Light particles each have energy E:
Here, h is Planck’s constant:
4.2 The Formation of Spectral Lines
The photoelectric effect :
• When light shines on metal, electrons can be emitted
• Frequency must be higher than minimum, characteristic of material
• Increased frequency – more energetic electrons
• Increased intensity – more electrons, same energy
• Einstein won his Nobel prize for explaining this effect (NOT for relativity theory)
4.2 The Formation of Spectral Lines
Photoelectric effect can be understood only if light behaves like particles
4.3 Molecules
Molecules can vibrate and rotate, besides having energy levels
• Electron transitions produce visible and ultraviolet lines
• Vibrational transitions produce infrared lines
• Rotational transitions produce MW & radio-wave lines
4.3 Molecules
Molecular spectra are much more complex than atomic spectra, even for hydrogen:
(a) Molecular hydrogen (b) Atomic hydrogen
(Band spectra) (Line spectra)
Astrophysical Molecular Spectra
• These next few slides show spectra of astronomical objects revealing the presence of common organic and inorganic molecules
“out there”
• These spectra are part of projects with our
NASA colleagues that Dr. Blass, myself and our students work on together
• They are infrared molecular spectra taken by the Voyager , Galileo , Cassini and other spacecraft as well as by ground based, airborne and balloon telescopes
water germane
50 20
Wavelength ( μm)
15 12 10 9
Methane
Phosphine
Ammonia
Hydrogen
Ammonia
Acetylene
Ethane
Wavenumber (cm -1 )
4.4 Spectral-Line Analysis
Information that can be gleaned from spectral lines:
• Chemical composition (Wavelength pattern)
• Temperature (Wien’s law)
• Radial velocity (Doppler effect): moving away source at rest (lab) moving toward
RED 650 nm 450 nm BLUE
4.4 Spectral-Line Analysis
Line widths can be due to Doppler (motional) broadening caused by
• thermal motion
• rotation
Or
From pressure broadening due to collisions altering the velocities ( Lorentzian broadening )
Linewidth
(FWHH)
4.4 Spectral-Line Analysis
Summary of Chapter 4
• Spectroscope splits light beam into component frequencies
• Continuous spectrum is emitted by solid, liquid, and dense gas
• Hot gas has characteristic emission spectrum
• Continuous spectrum incident on cool, thin gas gives characteristic absorption spectrum
Summary of Chapter 4, cont.
• Spectra can be explained using atomic models, with electrons occupying specific orbitals
• Emission and absorption lines result from transitions between orbitals
• Molecules can also emit and absorb radiation when making transitions between vibrational or rotational states