Chapter 4: Spectroscopy

Lecture II
Light spectra
The Birth of the Quantum
• Max Planck
– The energy contained in radiation is related to the frequency of the radiation by the relationship
E
nhf
• n is a positive integer called the quantum number
• f is the frequency of the oscillation
– A discreet packet of energy, later to become known as “a photon”
Implications of Planck’s Law
• The energy levels of the molecules must be discreet
• Only transitions by an amount E= hf are allowed
• The implication is that light is discreet or quantised energy
4 hf
3 hf
2 hf
1 hf
0 n
2
1
4
3
0
These quantum levels are known as number states
Spectroscope
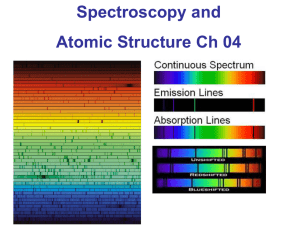
Three Types of Spectra
Spectral Analysis of the Elements
Studying the light emitted by an object in order to know something about that object!
Continuous Spectrum: a collection all possible wavelengths/ frequencies of light
Emission Spectra
Pattern of bright spectral lines produced by an element.
Absorption Spectra
Pattern of dark spectral lines where light within a number of narrow frequency ranges has been removed.
Wavelength
Bright Line Emission Spectra
Hydrogen
Helium
Argon
Neon
Krypton
Kirchoff’s Laws
• 1st law: A luminous solid or liquid, or a sufficiently dense gas, emits light of all wavelengths and produces a
continuous spectrum of radiation.
• 2nd law: A low-density hot gas emits light whose spectrum consists of a series of bright emission lines which are characteristic of the chemical composition of the gas.
• 3rd law: A cool thin gas absorbs certain wavelengths from a continuous spectrum, leaving dark absorption lines in their place superimposed on the continuous spectrum.
Spectra and Background
Type of spectrum seen depends on the temperature of the thin gas relative to the background temperature.
TOP: thin gas cooler than background, absorption lines seen.
BOTTOM: thin gas hotter than background, emission lines seen.
Studying the Stars:
Analyzing the light from a star can tell us:
1. The composition of the star.
2. The relative motion & rotation of the star.
3. The star’s temperature.
Shows limited Range of Light Energies Reaching Earth’s Surface