Spectra Lab Introduction

Spectra Lab
Introduction
It is important to understand the properties of light because most of an astronomer’s information about celestial objects arrives in the form of electromagnetic waves. Astronomers, both in the laboratory and at the telescope, have spent a great deal of time devising techniques to decode the message of light in order to yield as much information as possible. By examining a mere pinpoint of light we can now determine many of the physical properties of the body from which the light originates; properties such as: temperature and chemical composition to name a few.
Problem: What inferences can scientists make about the light coming from galaxies?
Research:
- What is the electromagnetic spectrum.
- list the types of radiation that make up the electromagnetic spectrum.
- What is the speed of all forms of energy as they travel through the
vacuum of space.
- What is a photon?
- What is spectroscopy?
Materials: spectrograms (strips of scale) crayons or colored pencils spectrometer various light sources
Part I (Various Burning Gases and Spectrum)
Observe the emission spectra of the following chemical salts demonstrated in class.
1.
List the salt being demonstrated
2.
Observe the general color and record
Data Entry:
Salts General Color
___________ _____________
___________ _____________
___________ _____________
Part II
1.
As a class observe the spectral tubes.
2. Record the spectral lines as seen on the Web site:
Jersey.uoregon.edu/vlab/elements/Elements.html mark the spectral lines as shown on the web site for each element
2013
Gases General Color
___________ ______________
___________ _____________
___________ _____________
Analysis: Answer the following questions using complete sentences, complete thoughts.
1.
Why are the spectrograms for the spectrum tubes of gas (Part II) different?
2.
What information does observing the spectral lines give us about these sources of light (energy)?
Part III - Continuous Spectrum
1. Use a spectrometer and observe the light in a classroom window. Using colored pencils, color in the spectrum you see.
2. Use a spectrometer and observe the spectrum of an incandescent light bulb. Color the spectrum you see.
Sunlight incandescent light bulb
3. Compare the spectrogram for sunlight with the spectrogram of the incandescent light bulb... Were they the same or different? Why?
2013
Part IV - Emission Spectra
1. Compare the spectrograms for florescent and Mercury Vapor lights. Explain .
Florescent Mercury Vapor
2. What are emission spectral lines and what do they tell us?
Part V - Absorption Spectral Lines
1. Explain how absorption lines are different than emission lines. (see textbook)
Part VI - more information
There is far more information in light spectra. Using a calculation called “Wien’s Law” you can measure the temperature of the light source (star). Using a calculation called “Stefan’s Law” you can measure the energy output and brightness of the light (star).
Even an approximation of a star’s age can be given. If a star is created by “slamming” the nucleus of two or more atoms together(nuclear fusion) and creating even bigger atoms of different elements then the more elements, the longer the star has been around. Elements are actually created in the energy of a burning star.
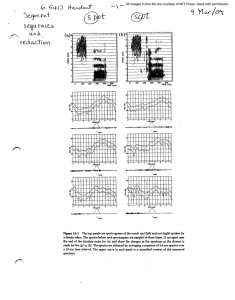
Below are photos of actual spectrograms of different stars.
Which star whose spectra is shown below might be the oldest? Explain why.
Stars: labeled as letters below
2013
Conclusion: Answer the PROBLEM, in a complete sentence, using the evidence that was gathered in this lab. write a 5 sentence paragraph (minimum)
Summarize what scientists infer from reading the spectra of the stars and galaxies?
2013