Chapter 2: Chemical Foundations for Cells
advertisement

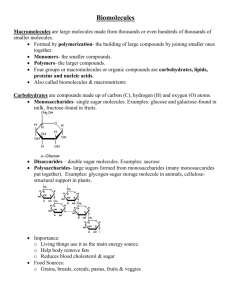
Chapter 2: Chemical Foundations for Cells Chapter 3: Carbon Compounds in Cells Elements are fundamental forms of matter (which is anything that has mass and takes up space). Naturally, elements cannot be broken apart (but can be in a physics lab). There are 92 naturally occurring elements, and four of them are abundant in natural systems: carbon, oxygen, hydrogen, and nitrogen. Our bodies also contain: Phosphorous - a mineral, helps build strong bones and teeth. Potassium - Potassium is a mineral that helps the kidneys function normally. It is also an electrolyte, a substance that conducts electricity in the body, along with sodium, chloride, calcium, and magnesium. Sulfur - Sulfur helps to maintain protein structural stability, participates in enzyme activity through tissue respiration, contributes to detoxification. Calcium - Mineralization of bones and teeth is the function of calcium that demands approximately 99% of the total body calcium. Sodium – Sodium is an absolutely necessary mineral for the human body. Without it, nerves and muscles would cease to function, the absorption of major nutrients would be impaired, and the body would not be able to maintain adequate water and mineral balance Chlorine - Chlorine is a part of hydrochloric acid, used to digest food. Plus some trace elements such as: Iodine - It is known to be essential in maintaining the function of the thyroid and parathyroid glands in the human body Iron - Iron is considered an essential mineral because it is needed to make part of blood cells. The human body needs iron to make the oxygen-carrying proteins hemoglobin and myoglobin. Magnesium - Magnesium is essential to the functioning of the human body because it transmits nerve impulses, causes the contraction of muscles and is integral to healthy development of teeth Elements bond together to form compounds or mixtures. Compounds – 2 or more different elements bonded together in proportions that never vary. Mixtures – 2 or more different elements bonded together in proportions that can, and generally do, vary. There are 4 groups of compounds crucial to life: carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates The most abundant of all biological molecules. Used as structural material and transportable or storable energy forms. Made of carbon, hydrogen, and oxygen in the ration 1:2:1 (CH2O)n. Three classes: monosaccharides, disaccharides, and polysaccharides. (saccharide meaning sugar) Monosaccharide Most simple of the carbs. Glucose Sweet tasting and readily dissolve in water. Backbone of five or six carbons. Form a ring in bodily fluids. Fructose Short Chain Carbohydrates Disaccharides (2) or oligosaccharides (few) Made of two sugar units. Sucrose is the most common natural sugar. Plants Convert sugar stores to sucrose for easy transport. Table sugar is the crystallized form of this. Sucrose Complex Carbohydrates Polysaccharides - chains of many monosaccharides (often hundreds of the same type). Examples: cellulose, starch, glycogen, chitin Cellulose – plant structural material (tough, insoluble) Glycogen – animal sugar storage. Housed largely in muscle and liver. Low blood sugar triggers its release. Starch – plant sugar storage. Chitin – nitrogen containing polysaccharide main structural component of insect external skeleton and outside wall of fungus. Lipids Made of hydrocarbons that don’t readily dissolve in water. Greasy or oily to touch. Used as a main energy reservoir, structural materials (cell membranes), signaling molecules. Fats – one two or three fatty acids attached to glycerol Fatty acids – backbone of up to 36 carbons with a carboxyl at one end, with hydrogens occupying most of the other bonding sites. Unsaturated – one or more double bonds – liquid at room temperature Saturated – no double bonds – solid at room temperature. Most animal fats are saturated. Triglycerides – natural fats – butter, lard. Three fatty acids attached to a glycerol. Body’s most abundant lipid. Richest energy source. Stored in body fat tissue (adipose). In some animals, it serves as insulation. (penguins). Phospholipids – Glycerol backbone, 2 fatty acids and a hydrophilic head of a phosphate group with a polar group. Main material of the cell membrane. Sterols – lipids with no fatty acids. Rigid barrier of four fused carbon rings. In eukaryotic cell membranes. Cholesterol is the most common. Cells use cholesterol remodeled into vitamin D, bile salts (digest fat), or steroids (sex hormones – estrogen, testosterone). Waxes – long fatty acid chains linked to long chain alcohols. Firm consistency. Repel water. Secreted to keep feathers dry. Beeswax in honeycombs. Amino Acids – most diverse of compounds Enzymes – speed up metabolic activities. Move molecules and ions across membranes (transport). Protein hormones – regulation of cell activities. Act as a weapon against disease causing agents. Build entire proteins from just 20 amino acids. Glycoproteins and Lipoproteins – Glyco form when the proteins bond to a sugar. Lipo form when the protein bonds to a fat. Glyco are used on the surface of animal cells. Many secretions form cells and proteins in blood contain both. Denaturation – Whenever a protein has its shape disrupted. Caused by pH or temperatures that exceed its range of tolerance. The chains will unwind or unfold and the protein loses its function. Example: frying an egg. In some cases, it is reversible when normal conditions return, but not in the case of the egg. Nucleotides and Nucleic Acids Nucleotides – small organic compounds with a sugar, a phosphate group and a base. The sugar is either ribose or deoxyribose. Both have a five carbon ring structure, but ribose has an oxygen attached that deoxyribose does not. ATP – three phosphates attached to its sugar. Transfers a phosphate to a molecule to release energy and help in metabolism. Nucleic acids – form single or double stranded molecules. DNA – made of a double helix that twists into a helix. Genetic information is coded on the DNA base sequences. RNA – Single stranded nucleotides. Use genetic information to build proteins. Water Without water, none of these things would work effectively. Organisms that don’t live in water still cart it around in their body. Metabolic reactions need it in many cases. Cell shape and structure depend on it. Water molecules have no net charge, but it is still an uneven compound. Its oxygen is slightly negative, which causes it to attract certain compounds and repel others. Hydrophilic – water loving. Readily bond with water. (Example: sugars) Hydrophobic – water fearing. They repel water. (Example: fats) Water has temperature-stabilizing effects. Cells are made mostly of water, and release a lot of heat during metabolism. Water can absorb more heat energy than any other fluids without suffering a rapid rise in temperature. In some cases, water molecules do escape to the air in evaporation. By escaping, they take excess heat and cool off the body (cell). Cohesion is the ability to resist breaking when put under tension. Hydrogen bonds result in excellent surface tension Because of this cohesion, water can move through vascular tissues easily. Solvent properties – water is a good solvent. Ions and polar molecules readily dissolve in it. This means that substances are surrounded by water and easily spread throughout it.