2014 Osmosis Practice - Ms Kim's Biology Class
Name ___________________________________________________ Date __________________________________ Period __________
Diffusion and Osmosis and Tonicity
1. What is diffusion?
2. What is facilitated diffusion?
3. What is osmosis?
4. In which direction does water move across membranes, up or down the concentration gradient? ______________________________
5. Define these 3 terms: a. isotonic- b. hypertonic c. hypotonic
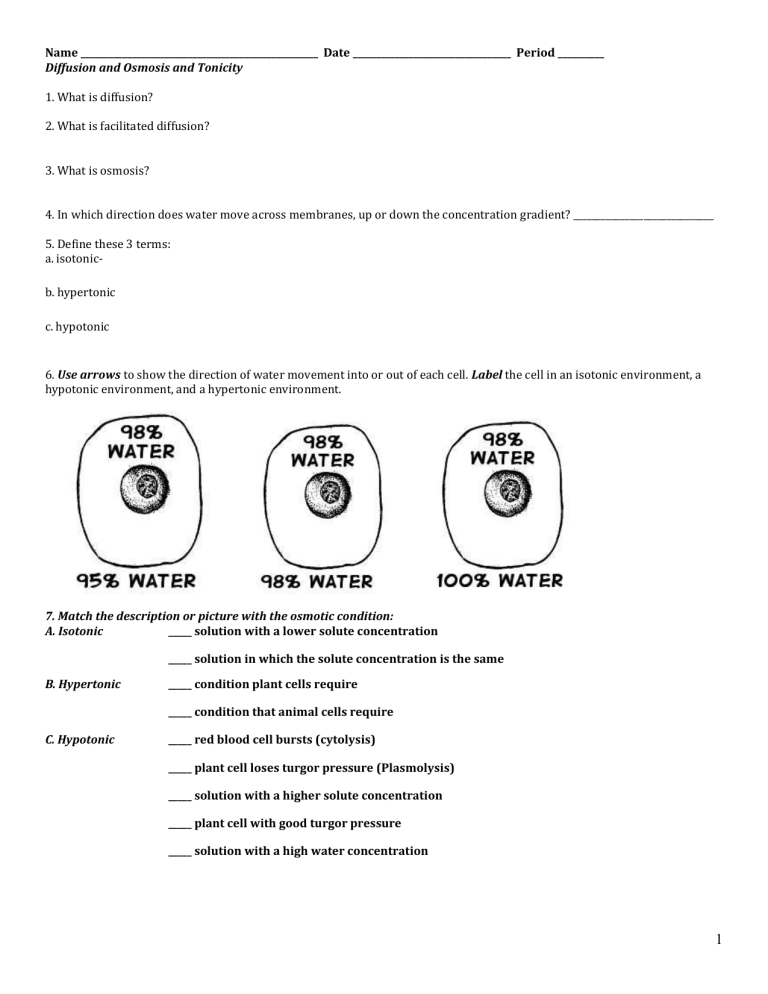
6. Use arrows to show the direction of water movement into or out of each cell. Label the cell in an isotonic environment, a hypotonic environment, and a hypertonic environment.
C. Hypotonic
7. Match the description or picture with the osmotic condition:
A. Isotonic _____ solution with a lower solute concentration
B. Hypertonic
_____ solution in which the solute concentration is the same
_____ condition plant cells require
_____ condition that animal cells require
_____ red blood cell bursts (cytolysis)
_____ plant cell loses turgor pressure (Plasmolysis)
_____ solution with a higher solute concentration
_____ plant cell with good turgor pressure
_____ solution with a high water concentration
1
8. Draw the arrow for the MOVEMENT OF WATER and LABEL the tonicity for each solution (isotonic, hypotonic, or hypertonic) Show solute:
9) Diffusion and Osmosis Problems:
1.) If a cell (with no dye in it) is in a solution of 10% dye molecules and the dye can cross the cell’s membrane, what do you predict will happen to the dye?
2.) If a cell (with 10% oxygen in it) is in a solution of 3% oxygen molecules and the oxygen can cross the cell’s membrane, what do you predict will happen to the oxygen?
3.) You have a cell, with a semi permeable membrane and a 1.5% potassium concentration. You put it into a solution of 2% potassium. What type of solution is it in? What direction would you expect water to flow? What do you expect to see happen to the cell?
4.) You have a cell, with a semi permeable membrane and a 1.5% potassium concentration. You put it into a solution of
1.05% potassium. What adjective describes the solution? What direction would you expect water to flow? What do you expect to see happen to the cell?
10.
Label the diagram below using the following: plasmolysis, cytolysis, healthy plant cell, healthy animal cell, turgid
11.
Observe the diagram below and answer the questions. HINT: what type of cell is this?
20% water
1 0%
salt a.. Can you tell if the cell is in a hypertonic, hypotonic, or isotonic solution? Why or why not? b. What will happen to the cell if it is placed in a 50% salt solution? c.. What will happen if the cell is placed in pure water?
2
12.
Observe the table below. Are the following hypotonic, hypertonic, or isotonic solutions? Which way will water mostly move? (some situations may have water moving equally) intracellular fluid
(CYTOPLASM ) extracellular fluid
(outside of the cell)
Hypotonic,
Hypertonic,
Isotonic? water moves mostly
inside or
outside the cell cell shrinks, swells, or stays the same size
5% salt 10% salt
10% salt
3% glucose
9% salt
13% water
10% salt
1% glucose
9% salt
25% water
59% water 45% water
74% glucose 87% glucose
13.
Consider this diagram.
(Permeable to glucose and water and Impermeable to protein) a) Will the concentration of water stay the
same on side A or become greater or less
with time?______________________ b) Will the concentration of protein on side
A stay the same or become greater or less
with time? _____________________ c) Glucose will cross the membrane in
which direction? __________________ d) Which side will rise? __________________ e) After the U-tube reaches equilibrium, what will the % of the level glucose on each side be? _____________________________
11. Red blood cells neither gain nor lose water when put into 0.9% NaCl. a) What term would you use to describe the tonicity of 0.9% NaCl for Red blood cells? _________ b) Are the solutions below hypertonic or hypotonic to red blood cells? i) 15% NaCl __________________ ii) 0.001% NaCl __________________
14) Answer True or False a. If a plant cell is placed in salt solution, the central vacuole will shrink b. If a red blood cell is placed in distilled water, it will shrink c. If a plant cell is placed in distilled water, the cell membrane will move away from the cell wall d. If a red blood cell is placed in a salt solution, salt will enter the cells, giving them a strange appearance e. Crenation is to plasmolysis as hemolysis is to turgor pressure
3
15.
Observe the diagrams in the table below. Assume that the dots are dissolved particles on either side of the cell membrane. They are like oxygen molecules that can go across the membrane. Do the following situations represent concentration gradients? If so, in which direction would diffusion occur?
\
[ ] gradient?
Yes or No movement
left, right, or
none
[ ] gradient?
Yes or No movement
left, right, or none
[ ] gradient?
Yes or No? movement
left, right, or
none
16.
Observe the diagrams in the table below. Assume that the dots are dissolved particles (like
protein or carbohydrate molecules) on either side of the cell membrane. Do the following situations represent concentration gradients? If so, in which direction would osmosis occur?
[ ] gradient?
Yes or No movement
left, right, or
none
[ ] gradient?
Yes or No movement
left, right, or none
[ ] gradient?
Yes or No? movement
left, right, or
none
17) Read the following questions carefully. Be sure to answer all parts of each question.
1. If a cell contains 85% water and is placed into a environment which is 50% water. Is the cell hypotonic or hypertonic? In which direction will the water move, into the cell or out of the cell?
2. A cell contains 85% water and is placed into a solution which contains 90% water. Is the solution in the environment hypotonic or hypertonic? In which direction will the water move?
3. If a cell which contains 90% water is placed into an isotonic solution, what percentage of the solution is water?
4. A cell contains 95% water, the environment outside the cell contains 45% solutes. Is the cell in a hypotonic or hypertonic environment?
5. A cell containing 25% solutes is placed into a solution which contains 35% solutes. Which direction will the water move, into the cell or out of the cell?
6. A plant cell containing 95% water is placed into a 10% salt solution. Is the salt solution hypotonic or hypertonic? Which direction will the water move?
4
7. What types of molecules have difficulty crossing the plasma membrane? Why?
8.
What is meant by a concentration gradient?
9.
A cell whose internal osmotic concentration is 0.3 osmoles/liter is placed in a solution that is 0.5 osmoles/liter. The solution is ___________________ relative to the cell.
18) DIAGRAM BELOW: The solutions in the two arms of this U-tube are separated by a membrane that is permeable to water and glucose but not to sucrose. Side A is filled with a solution of 2.0 M sucrose and 1.0 M glucose. Side B is filled with a solution of 1.0 M sucrose and 2.0 M glucose.
A.
Initially, the solution in side A is _____________________ with respect to that in side B.
B.
After the system reaches equilibrium, describe what changes are observed.
19) A semipermeable sac, containing 4% NaCl, 9% glucose, and 10% albumin, is suspended in a solution with the following composition: 10% NaCl, 10% glucose, and 40% albumin. Assume the sac is permeable to all substances except albumin. Use the key provided to determine how each substance will move.
A. Moves into the sac B. Moves out of the sac C. Does not move
______ Glucose ______ Albumin ______ Water ______ NaCl
20) The diagrams below show three microscopic fields (A-C) containing red blood cells. Arrows indicate the direction of net water movement. Answer the following questions. a.
Which microscopic field shows a hypertonic environment?__________ b.
Which microscopic field shows a cell hypertonic to its environment? _______________ c.
Which microscopic field shows an isotonic environment?____________ d.
Describe what is happening to the cells in Field C: e.
Why is this happening?
5
21) . Examine the drawing below. The tube, filled with 10% sucrose solution, was placed in a 5% sucrose solution. The membrane covering the bottom of the tube is impermeable to sucrose. a. In what direction does water move? _____________________________ b. Explain why in terms of osmosis: c. Are the tube contents hypertonic, hypotonic, or isotonic? _________________ d. Is the solution outside the tube hypertonic, hypotonic, or isotonic? _________________
22. An artificial cell with an aqueous solution enclosed in a selectively permeable membrane has just been placed in an environment containing a different solution. The membrane is permeable to water and to the simple sugars glucose and fructose, but is completely impermeable to sucrose. Use this information and the diagram at the right to answer the questions that follow. a. Which solute(s) will exhibit a net diffusion into the cell? ________________________________ b. Which solute(s) will exhibit a net diffusion out of the cell? _________________________________ c. In which direction will there be a net osmotic movement of water? ___________________________ d. After the cell is placed into the environment, which of the following changes would occur? (Check the correct one.)
________The artificial cell would be become flaccid. _______The artificial cell would become more turgid.
_______There would be no net change in the cell.
6







