Chemistry Name: Unit 8 Related Problems Date Due: Perform the
advertisement
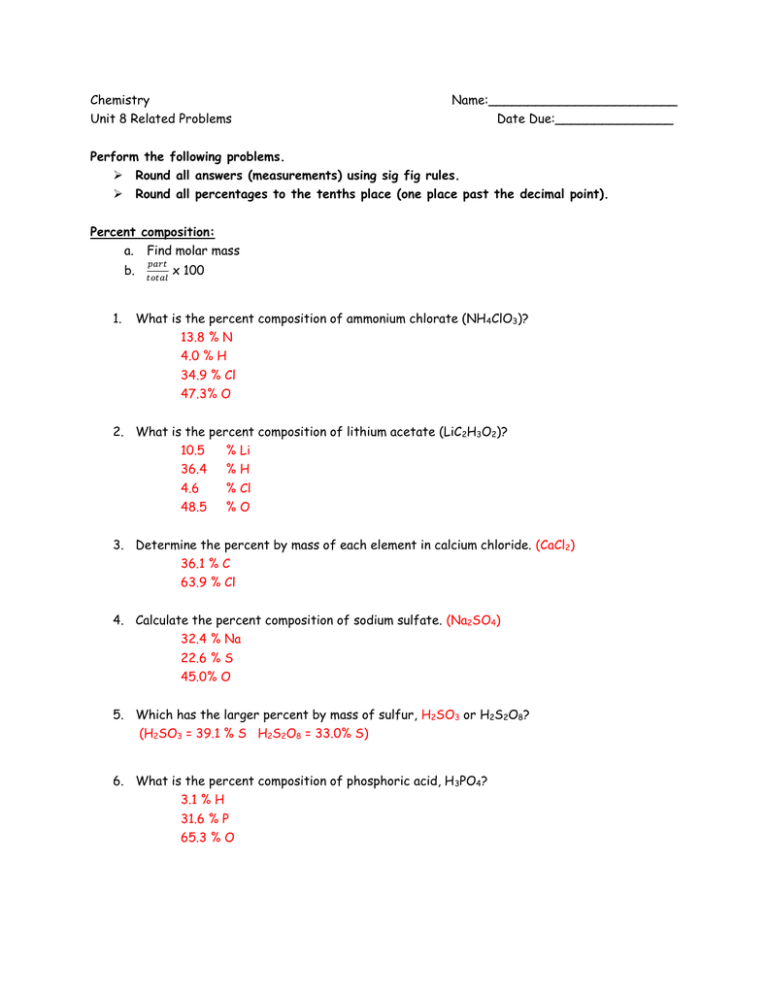
Chemistry Name:________________________ Unit 8 Related Problems Date Due:_______________ Perform the following problems. Round all answers (measurements) using sig fig rules. Round all percentages to the tenths place (one place past the decimal point). Percent composition: a. b. 1. Find molar mass 𝑝𝑎𝑟𝑡 𝑡𝑜𝑡𝑎𝑙 x 100 What is the percent composition of ammonium chlorate (NH 4ClO3)? 13.8 % N 4.0 % H 34.9 % Cl 47.3% O 2. What is the percent composition of lithium acetate (LiC2H3O2)? 10.5 % Li 36.4 %H 4.6 % Cl 48.5 %O 3. Determine the percent by mass of each element in calcium chloride. (CaCl2) 36.1 % C 63.9 % Cl 4. Calculate the percent composition of sodium sulfate. (Na2SO4) 32.4 % Na 22.6 % S 45.0% O 5. Which has the larger percent by mass of sulfur, H2SO3 or H2S2O8? (H2SO3 = 39.1 % S H2S2O8 = 33.0% S) 6. What is the percent composition of phosphoric acid, H 3PO4? 3.1 % H 31.6 % P 65.3 % O We will do this section time permitting… Using percent composition and ratios: 7. How many grams of phosphorus are present in 8.907 grams of Ba3(PO4)2? 8. How many grams of iodine are present in 75.3 gram sample of Zn(IO 3)2? 9. How many kilograms of mercury are present in 6.900 x 10 9 grams of (Hg2)3N2? 10. How many grams of carbon are present in a 4.000 grams sample LiCN? 11. How many grams of acetate (C2H3O21-) are there in a 33 gram sample of AgC2H3O2? 12. How many grams of hydroxide (OH1-) are present in a 196.00 sample of NH4OH? Empirical and Molecular Formulas 13. Find and use a pattern in 1-5 to fill in6-10 in the second table: Examples: Compound Molecular formula Empirical Formula 1 benzene C6H6 CH 2 hydrogen peroxide H2O2 HO 3 sodium thiosulfate Na2S2O3 Na2S2O3 4 potassium oxalate K2C2O4 KCO2 5 ribose C5H10O5 CH2O 14. What pattern do you notice? Reduced by GCF! 15. Compound Molecular formula Empirical Formula 6 dinitrogen tetraoxide N2O4 NO2 7 beta-carotene C40H56 C5H7 8 TNT C7H5N3O6 C7H5N3O6 9 tribenzoin C24H20O6 C12H10O3 10 caffeine C8H10N4O2 C4H5N2O Definitions: 16. Molecular Formula: the actual formula of the compound 17. Empirical Formula: reduced to the smallest whole number ratio of elements in a compound Empirical Formulas: a. assume 100 g sample = chage % to g (if necessary) b. use atomic masses to convert from grams to moles c. find mole ratio (divide by smallest number) d. write formula using numbers as a subscript 18. A blue solid is found to contain 36.84% nitrogen and 63.16 oxygen. What is the empirical formula for this solid? (N2O3) 19. Determine the empirical formula for a compound that contains 35.98% aluminum and 64.02% sulfur. (Al2S3) 20. Propane is a hydrocarbon, a compound composed only of carbon and hydrogen. It is 81.82% carbon and 18.18% hydrogen. What is the empirical formula? (C3H8) 21. The chemical analysis of aspirin indicates that the molecule is 60.00% carbon, 4.44% hydrogen and 35.56% oxygen. Determine the empirical formula. (C9H8O4) 22. What is the empirical formula of a compound if it contains 29.1% Na, 40.5% S, and 30.4% O? (Na2S2O3) 23. A compound was analyzed in a lab to determine its empirical formula. Decomposition of the compound at standard temperature and pressure produced 9.00 g of carbon, 16.8 L of hydrogen and 2.80 L of oxygen. What is the empirical formula of this compound? use molar volume (C6H6O) Molecular Formulas: a. same as empirical b. find the molar mass of the empirical formula c. divide: 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑎𝑟 𝑚𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑒𝑚𝑝𝑖𝑟𝑖𝑐𝑎𝑙 d. rewrite formula as multiple of empirical 24. Find the molecular formula for compound that contains 4.90 g nitrogen and 11.2 g oxygen. The molar mass of the compound is 92.0 g/mol. (N2O4) 25. Experimental analysis of the sweetener, mannitol, has determined that it contains 39.60% carbon, 7.75% hydrogen and 52.70% oxygen. The molecular mass is 182.20 g/mol. Determine the empirical and molecular formulas. (C3H7O3, C6H14O6) 26. The organic compound medicagenic acid is a synthetic fatty acid whose molar mass is 502.67 g/mol. Ana analysis of a sample of the compound determined that it contains 47.47 g carbon, 6.15 g hydrogen and 12.72 g of oxygen. Write the empirical and molecular formula for medicagenic acid. (C15H26O3, C30H52O6)