Pathology of chronic obstructive airway diseases
advertisement

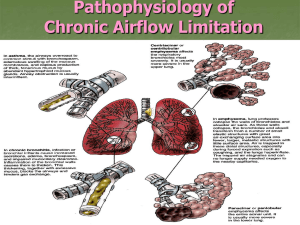
Pathology of chronic obstructive airway diseases Definition • Chronic obstructive pulmonary disease (COPD) is defined as ‘a disease state characterized by airflow limitations that is not fully reversible. The airflow limitation is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gases’ (Global Initiative for Obstructive Lung Disease: GOLD). Two Ways to Limit Airflow 1. Reduced outflow pressure due to loss of elastic recoil (emphysema) 2. Increased airway resistance (chronic bronchitis, bronchiolitis) Three Obstructive Airway Diseases • emphysema (destruction of air spaces and loss of elastic recoil) • chronic bronchitis (mucus hypersecretion and luminal narrowing of airways) • bronchiolitis (narrowing of small airways by inflammation and fibrosis) Emphysema: Protease-antiprotease imbalance • Nicotine and reactive oxygen species attract neutrophils into alveoli • Cigarette smoke activates NF-KB transcription factor -> increased TNF & IL-8 attracting and activating neutrophils. Neutrophil elastase, proteinase, cathepsin-G is released • Reactive oxygen species inactivate α1-antitrypsin causing functional α1-antitrypsin deficiency • Neutrophil destruction of alveolar and respiratory bronchiolar walls, as well as the elastic tissue within the alveolar septa occurs unabated in the absence of α1-antitrypsin • Macrophages, present in greater numbers, can release their own elastase which is normally not inhibited by α1-antitrypsin Emphysema: protease-antiprotease imbalance and oxidant-antioxidant imbalance Enlargement of airspaces and thinning of alveolar septa in emphysema Types of Emphysema • (B & C) Centriacinar (D&E) Panacinar Bronciolitis • Inflammation, caused by macrophage and lymphocyte infiltration of the small bronchiole (< 2mm) walls, is an early sign of functional airway obstruction caused by smoking Chronic Bronchitis • Long term inhalation of cigarette smoke and/or air pollution (nitrogen dioxide, sulfur dioxide) causes increased transcription of the mucin gene resulting in: – Hypertrophy of mucus glands in trachea and main branch bronchi causing a chronic productive cough – Metaplasia of goblet cells in small bronchi and bronchioles causing mucus plugging – inflammation with infiltration of CD8+ T cells, macrophages, and neutrophils (differentiated from asthma by lack of eosinophils) causing damage, permanent lumen narrowing and bronchiole wall thickening by fibrosis and ultimately closure (bronchiolitis obliterans) – Often accompanied by emphysema – Chronic respiratory failure develops (compensated by kidneys (bicarb retention & EPO secretion -> normal pH & polycythemia) – Recurrent microbial infections exacerbate the symptoms, accelerate the course of the disease and cause bouts of acute (uncompensated) respiratory failure requiring emergency treatment Chronic bronchitis showing doubling of thickness of mucus gland layer of a bronchus Chronic Respiratory Failure • Acute hypercapnic respiratory failure is compensated by buffering only because it rapidly develops over minutes to hours; therefore, pH is less than 7.3 (respiratory acidosis) • Chronic respiratory failure develops over several days or longer, allowing time for renal compensation and bicarbonate retention. Therefore, the pH usually is only slightly decreased. • The distinction, however, between acute and chronic hypoxemic respiratory failure should not be made on the basis of ABGs but rather by clinical markers of such as polycythemia or cor pulmonale, suggesting a long-standing disorder Quick Review of Pathology of Asthma Changes To The Bronchiole Walls Due To Asthma