Topic 5.1 Measuring Energy Changes
advertisement
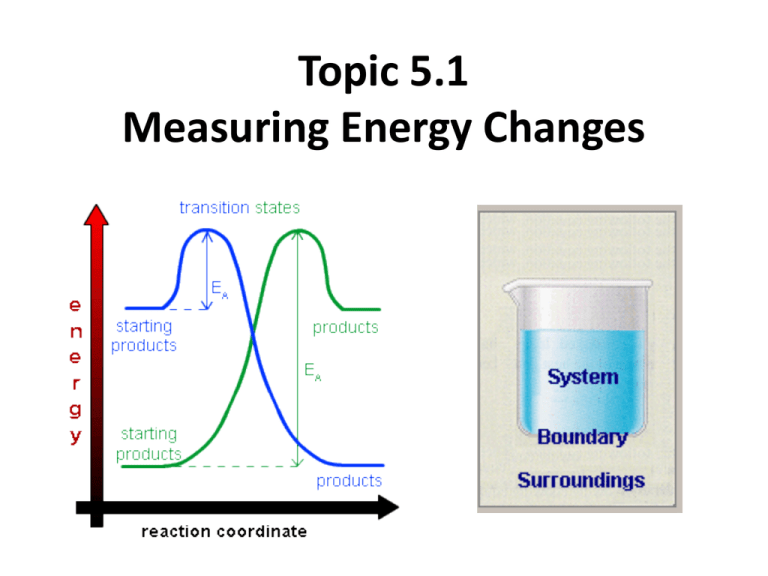
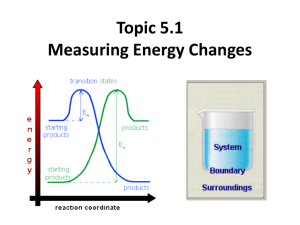
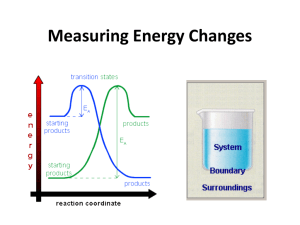
Topic 5.1 Measuring Energy Changes • the conservation of energy is a fundamental principle of science – if a system loses energy, it must be gained by the surroundings, and vice versa • when examining energy changes in a chemical reaction, we divide “the universe” into two parts Temperature vs. Heat • temperature – a measure of the average kinetic energy of the particles regardless of the amount • °C or K • heat (q) – a form of energy that is transferred from a warmer body to a cooler one. – measures the total energy in a given substance (amount does matter) Enthalpy H • the amount of heat change (put into or released) in a system • you cannot measure the actual enthalpy (energy) of a substance directly – but you can measure an enthalpy CHANGE because of energy it takes in or releases • enthalpy is an example of a state function – does not depend on the path taken to reach its present state or value – the value is independent of changes “along the way” – only the beginning and end matter – ΔTemp = Temp(final) - Temp(initial) Standard enthalpy change of formation vs • use the symbol • energy change needed to make 1 mole of a substance • a pure substance under these conditions is said to be in its standard state –pressure: 100 kPa –temperature: 298K (25°C) • example- using the formation of phenol – 6C(s) + 3H2(g) + ½ O2 1C6H5OH(s) – ΔHɵ = -165.0 kJ mol-1 Enthalpy (Heat) of a Reaction • the amount of energy absorbed or given off in a chemical reaction • H = ∑ Hproducts − ∑ Hreactants Exothermic reactions • heat energy is given out by the reaction hence the surroundings increase in temperature (feels hot) • occurs when bonds are formed – new products are more stable and extra energy is given off • Hproducts < Hreactants – H is negative • examples – combustion of fuels – respiration – neutralization reactions (acid reacts with something) ENTHALPY activation energy energy necessary to get the reaction going energy given out, ∆H is negative reactants products REACTION CO-ORDINATE (or time) H2 + Cl2 2HCl Energy taken in to break bonds. H, H, Cl, Cl (Atoms) Energy given out when bonds are made. energy H-H, Cl-Cl Reactants Overall energy change, H H-Cl, H-Cl Products H2 + Cl2 2HCl Energy in = +678kJ H, H, Cl, Cl (Atoms) Energy out = -862kJ energy H-H, Cl-Cl Reactants Overall energy change, H = -184kJ H-Cl, H-Cl Products Endothermic reactions • heat energy is taken in by the reaction mixture hence the surroundings decrease in temperature (feels cold) • occurs when bonds are broken – the reactants were more stable (bonds are stronger) • overall, took energy from the surroundings • Hreactants < Hproduct H is positive • examples – photosynthesis activation energy energy necessary to get the reaction going ENTHALPY products reactants REACTION CO-ORDINATE Energy taken in and now stored in the bonds. ∆H is positive Summary Table Exothermic reactions Endothermic reactions Energy is given out to the surroundings Energy is taken in from the surroundings ∆H is negative ∆H is positive Products have less energy than reactants Products have more energy than reactants Calculation of enthalpy change Heat Capacity/Specific Heat • the amount of energy a substance absorbs depends on: – mass of material – temperature – kind of material and its ability to absorb or retain heat • heat capacity – the amount of heat required to raise the temperature of a substance 1 oC (or 1 Kelvin) • molar heat capacity – the amount of heat required to raise the temperature of one mole 1 oC (or 1 Kelvin) • specific heat – the amount of heat required to raise the temperature of 1 gram of a substance 1 oC (or 1 Kelvin) Specific Heat (c) values for Some Common Substances Substance J g-1 K-1 Water (liquid) 4.184 Water (steam) 2.080 Water (ice) 2.050 Copper 0.385 Aluminum 0.897 Ethanol 2.44 Lead 0.127 or kJ kg-1 K-1 if multiply by 1000 23 Heat energy change/transfer q = m c T • q = change in heat (same as H if pressure held constant) • m = mass in grams or kilograms • c = specific heat in J g-1 K-1 or kJ kg-1 K-1 (or Celsius which has same increments as Kelvin) • T = temperature change Measuring the temperature change in a calorimetry experiment can be difficult since the system is losing heat to the surroundings even as it is generating heat. By plotting a graph of time vs. temperature it is possible to extrapolate back to what the maximum temperature would have been had the system not been losing heat to the surroundings. Heat Transfer Problem 1 Calculate the heat that would be required to heat an aluminum cooking pan whose mass is 402.50 grams, from 20.5oC to 201.5oC. The specific heat of aluminum is 0.902 J g-1 oC-1. q = mcT only 4 sig. figs. = (402.50 g) (0.902 J g-1 oC-1)(181.0oC) = 65,712.955 J = 65,710 J with correct sig. figs. Heat Transfer Problem 2 What is the final temperature when 50.15 grams of water at 20.5oC is added to 80.65 grams water at 60.5oC? Assume that the loss of heat to the surroundings is negligible. The specific heat of water is 4.184 J g-1 oC-1 Solution: q (cold) = q (hot) so… mCT = mCT Let Tf = final temperature of the water (50.15 g) (4.184 J g-1 oC-1)(Tf - 20.5oC) = (80.65 g) (4.184 J g-1 oC-1)(60.5oC - Tf) (50.15 g)(Tf - 20.5oC) = (80.65 g)(60.5oC- Tf) 50.15Tf – 1030 = 4880 – 80.65Tf 130.80Tf = 5910 Tf = 45.2 oC Heat Transfer Problem 3 On complete combustion, 0.18g of hexane raised the temperature of 100.5g water from 22.5°C to 47.5°C. Calculate its enthalpy of combustion in kJ mole -1 of hexane. • Heat absorbed by the water… • q = mcT • q = 100.5 (4.18) (25.0) = 10,500 J which is same as 10.5 kJ – Moles of hexane burned = mass / molar mass = 0.18 g / 86 g/mol = 0.0021 moles of hexane hexane is C6H14 – Need to find heat energy / mole = 10.5 kJ/ 0.0021 mol = 5000 kJ mol -1 or 5.0 x 103 kJ mol -1