Measuring Energy Changes
advertisement

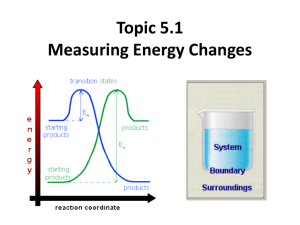
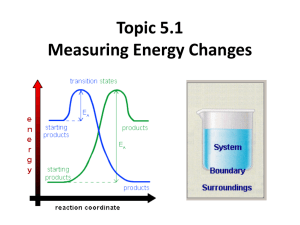
Measuring Energy Changes • total energy of the universe is a constant • law of conservation of energy – if a system loses energy, it must be gained by the surroundings, and vice versa • when examining energy changes in a chemical reactions, we divide “the universe” into two parts Temperature vs. Heat • temperature – a measure of the average kinetic energy of the particles regardless of the amount • heat (q) – one of many forms of energy – the transfer from a warmer body to a cooler body as a result of a temperature gradient – measures the total energy in a given substance (amount does matter) • 50 ml water 100 ml water • 100 C 100C 100ml of water contains twice the heat of 50 ml. Enthalpy H • the amount of heat used or released (change) in a system • you cannot measure the actual enthalpy of a substance directly – but you can measure an enthalpy CHANGE because of energy it takes in or releases Enthalpy (Heat) of a Reaction • the amount of energy absorbed or given off in a chemical reaction • H = ∑ Hproducts − ∑ Hreactants Exothermic reactions • heat energy is given out by the reaction hence the surroundings increase in temperature (feels hot) • occurs when bonds are formed – new products are more stable and extra energy is given off • Hproducts < Hreactants – H is negative • examples – combustion of fuels – respiration – neutralization reactions (acid reacts with something) ENTHALPY activation energy energy necessary to get the reaction going energy given out, ∆H is negative reactants products REACTION CO-ORDINATE H2 + Cl2 2HCl Energy taken in to break bonds. H, H, Cl, Cl (Atoms) Energy given out when bonds are made. energy H-H, Cl-Cl Reactants Overall energy change, H H-Cl, H-Cl Products H2 + Cl2 2HCl Energy in = +678kJ H, H, Cl, Cl (Atoms) Energy out = -862kJ energy H-H, Cl-Cl Reactants Overall energy change, H = -184kJ H-Cl, H-Cl Products Endothermic reactions • heat energy is taken in by the reaction mixture hence the surroundings decrease in temperature (feels cold) • occurs when bonds are broken – the reactants were more stable (bonds are stronger) • overall, took energy from the surroundings • Hreactants < Hproduct H is positive • examples – photosynthesis activation energy energy necessary to get the reaction going ENTHALPY products reactants REACTION CO-ORDINATE Energy taken in and now stored in the bonds. ∆H is positive Summary Table Exothermic reactions Endothermic reactions Energy is given out to the surroundings Energy is taken in from the surroundings ∆H is negative ∆H is positive Products have less energy than reactants Products have more energy than reactants Calculation of enthalpy change Heat Capacity/Specific Heat • the amount of energy a substance absorbs depends on: – mass of material – temperature – kind of material and its ability to absorb or retain heat • specific heat – the amount of heat required to raise the temperature of 1 gram of a substance 1 oC (or 1 Kelvin) 19 Specific Heat (c) values for Some Common Substances Substance J/g (K) Water (liquid) 4.184 Water (steam) 2.080 Water (ice) 2.050 Copper 0.385 Aluminum 0.897 Ethanol 2.44 Lead 0.127 or kJ/kg (K) if multiply by 1000 Heat energy change/transfer q = m c T • q = change in heat (same as H if pressure held constant) • m = mass in grams or kilograms • c = specific heat in J/g (K) or kJ/kg (K) (or Celsius which has same increments as Kelvin) • T = temperature change Heat Transfer Problem 1 Calculate the heat that would be required to heat an aluminum cooking pan whose mass is 402.50 grams, from 20.5oC to 201.5oC. The specific heat of aluminum is 0.902 J/g (oC) q = mcT = (402.50 g) (0.902 J/g (oC))(181.0oC) = 65,712.955 J = 65,710 J with correct sig. figs. only 4 sig. figs. Heat Transfer Problem 2 On complete combustion, 0.18g of hexane raised the temperature of 100.5g water from 22.5°C to 47.5°C. Calculate its enthalpy of combustion in kJ/mole of hexane. – Heat absorbed by the water… • q = mcT • q = 100.5 (4.18) (25.0) = 10,500 J which is same as 10.5 kJ – Moles of hexane burned = mass / molar mass = 0.18 g / 86 g/mol = 0.0021 moles of hexane – Need to find heat energy / mole = 10.5 kJ/ 0.0021 mol = 5000 kJ mol -1 or 5.0 x 103 kJ/mol hexane is C6H14