Nutrition Notes
advertisement

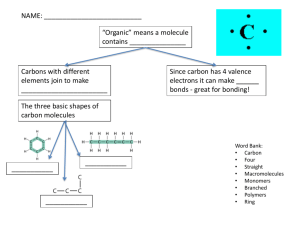
Nutrition Chapter 49-1 and Chapter 3 Unit 2 Lecture 4 Topic: Introduction to Organic Chemistry and Nutrition Covers: Chapter 3, pages 52 – 54 Chapter 49, page 977 All compounds can be classified in two broad categories: ORGANIC and INORGANIC compounds. Organic Compounds Molecules that contain carbon, hydrogen and oxygen The chemistry of carbon is considered to be “The chemistry of life”. In organic compounds, carbon atom is usually bonded to another carbon, hydrogen or oxygen Carbon is the “backbone” of organic compounds Carbon atoms can form 4 covalent bonds in all directions Can form many different shaped molecules – straight chain, branched chain, rings, etc NOTE: Bond represented by a line connecting Carbon to another element LARGE CARBON MOLECULES MONOMER - a single organic molecule Example: Glucose (blood sugar) POLYMER - two or more monomers together Example: Sucrose MACROMOLECULE - large organic molecule, made up of many polymers Examples: Glycogen, Starch Six Basic Food Ingredients All of the foods in the would contain at least one of six basic ingredients, also known as nutrients: Carbohydrates, lipids, proteins, vitamins, minerals, water Four of these nutrients are organic compounds Carbohydrates, lipids, proteins, vitamins Two of these nutrients are inorganic compounds Minerals, water These do not contain carbon, hydrogen and oxygen End of Lecture 4 Unit 4 Lecture 5 Topics: Carbohydrates and Lipids (Fats) Covers: Chapter 3, page 55 – 56 and 58 – 59 Chapter 49, page 977 – 979 Carbohydrates Made up of carbon, hydrogen and oxygen Function: Gives the body a quick energy source Easy for the body to break down carbs and convert into ATP MONOSACCHARIDE MONOMER of carbohydrate, aka Simple Sugar EXAMPLES: Glucose (blood sugar) Fructose (found in fruits, sweetest) Galactose (found in milk) Isomers – Molecules with same chemical formula but different structure CH2OH CH2OH O H OH O HO H CH2OH H O HO OH OH H OH OH GLUCOSE HO OH H OH OH OH GALACTOSE FRUCTOSE DISACCHARIDE 2 Monosaccharides combine to form a DISACCHARIDE, aka Double Sugar EXAMPLE: Sucrose (table sugar) = Fructose + Glucose Maltose (malt sugar) = Glucose + Glucose Lactose (milk sugar) = Glucose + Galactose POLYSACCHARIDE Many Monosaccharides combine to form a POLYSACCHARIDE EXAMPLES: Glycogen - many molecules of glucose How animals store glucose, good source of energy Stored in our liver and muscles Starch - many molecules of glucose How plants store glucose Cellulose - a form of starch, makes up the rigid cell wall We cannot digest cellulose, but it does stimulate smooth muscle contractions within the digestive system Lipids Lipids Made up of Carbon, Hydrogen, Oxygen Large molecules, long carbon “tail” Function: Used to build cell membranes, protect organs and provide insulation Gives the body an energy storage Lipids don't dissolve in water (NONPOLAR) Carbs that aren't converted into ATP will be stored as lipids Lipids are necessary to all living organisms Types of Lipids 1. Saturated Fats Can increase levels of bad cholesterol and blood cholesterol (bad) and decreases levels of good cholesterol Saturated fatty acids are usually solid at room temp EXAMPLES: Butter, Animal fat, Lard, Shortening Types of Lipids 2. Unsaturated Fats Can decrease levels of bad cholesterol and blood cholesterol Can increase levels of good cholesterol Unsaturated fatty acids are usually liquid at room temp. EXAMPLES: Olive oil, Plant seeds and fruits Some Types of Lipids: 3. Phospholipid Make up the cell membrane 4. Wax Forms a waterproof, protective coating Examples: ear wax, bees' wax, surface of plants End of Lecture 5 Unit 4 Lecture 6 Topics: Proteins, Vitamins, Minerals, Water Covers: Chapter 3, pages 56 – 57 Chapter 49, pages 977 – 982 Proteins Proteins Made up of Carbon, Hydrogen, Oxygen and Nitrogen Functions/Types of Proteins: Major source of structural material in the body Make up skin and muscles of animals Help body to grow and repair damaged tissue Some types of proteins: hormones, insulin, antibodies, enzymes, hair, skin pigment Proteins Proteins Proteins are macromolecules Made up of monomers known as AMINO ACIDS 20 different kinds of amino acids Every amino acid has the same basic structure EXCEPT for one part, known as the "R group” Each amino acid has a different R group Our body can't produce all 20 amino acids, although we need all 20 to function We get these 8 (or 10 for children) essential amino acids from our diet ENZYMES ENZYMES are proteins with a special job Name of enzyme usually ends in –ase (Ex: Sucrase) CATALYST - speed up the reactions in the body by lowering the activation energy Enzyme reactions depend on the physical fit between the enzyme and the substrate ENZYMES Enzyme and substrate have a specific form to allow them to fit together (like a lock and key) After the reaction is complete, the enzyme’s original shape returns This allows enzymes to be used numerous times Proteins Proteins are very large molecules made up of a long chain of amino acids Order and type of amino acids is different for each type of protein This gives each type of protein a different shape If the protein changes its form, it changes the function EXAMPLES: Egg whites, Enzymes The form of proteins can change because of temperature, amino acid sequence, incorrect folding VITAMINS Organic compound, nutrient, necessary for all living organisms Function: work as coenzymes Def: molecule that helps enzymes to be more efficient Can be used many times, just like enzymes This is why we only need a small amount of daily vitamins Our body can't make most vitamins Need to get vitamins from another source (food, supplements) Vitamins can be water or fat soluble If intake too many vitamins: Water soluble - released in urine Fat soluble - build up in body, can be fatal MINERALS Inorganic compound, nutrient, necessary for all living organisms Function: Provide necessary material needed for cells to function properly Our body can't make minerals Need to get minerals from another source (food, supplements) WATER Inorganic molecule, nutrient, necessary to maintain life Over half of your body weight is from water! Function: Regulate body temperature Dissolves substances (salts, sugars, wastes) Transportation of substances through cells and whole body Need to intake as much water (or more) than we lose during the day If losing too much water, cells won’t be able to function Known as Dehydration End of Lecture 6