Ionisation Energy Answers File
advertisement

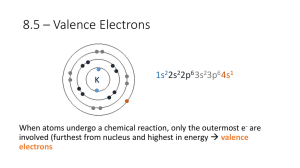
CHEMISTRY 3.4 WORKSHEET ONE ANSWERS ATOMS AND IONS Ionisation Energy 1. The energy required to remove an electron from one mole of gaseous atoms or ions. 2. kJ mol-1 3. (a) Na(g) → Na+(g) + e- (b) In order to remove electrons from atoms or ions, energy must be supplied. (a) As we remove electrons, the ion becomes successively more positive and the attractive forces holding the remaining electrons increase. The greater the force to overcome, the greater the energy required. (b) Removing the first three electrons requires them to be all taken from the third energy level. To remove the 4th electron requires one to be taken from the full second energy level which is at a significantly higher energy than the third level. (c) Lithium and sodium are both in group one and have only one valence electron to remove. However sodium has 3 energy levels and so its valence electron is further from the nucleus than lithium which has only 2 energy levels. This means that sodium’s valence electron is more easily removed than lithium’s. (d) Chlorine has 17 electrons. The graph shows that the first 7 are removed with steady but small increases in I.E. Then there is a big jump to the next 8 electrons and another big jump to the last 2. This suggests that the electrons are arranged in 3 energy levels. 2 electrons in the first level, 8 in the second level and 7 in the third level. (e) Filled and half filled energy levels have extra stability. Be and Mg both have their valence electrons in a filled s-shell. N and P both have their valence electrons in a half filled p-subshell. 4. ∆H = +494 kJ mol-1