How to draw Lewis diagrams for compounds
advertisement

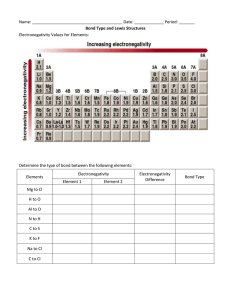
Lewis Structures Drawing Lewis Structures: 1. Draw skeletal structure of compound showing what atoms are bonded to each other. A) Put least electronegative element in the center. B) Use only single dashes C) Draw symmetrically. 2. Count total number of valence e-. Add 1 for each negative charge. Subtract 1 for each positive charge. From this total, subtract the electrons that are involved in bonding (2 electrons per dash) 3. Distribute the electron dots around the molecule symmetrically. 4. If octet rule is not being met, form double and triple bonds on central atom as needed. 5. Check your answer. A) Are all atoms meeting octet rule? B) Do the total number of electrons equal your original amount? If not, try again. Draw Lewis Structures for the following compounds. 1. F2 6. CO 2. H2O 7. CO32- 3. SCl2 8. SO42- 4. AsF3 9. N2F2 5. SiH4 10. C3H6O