slides
advertisement
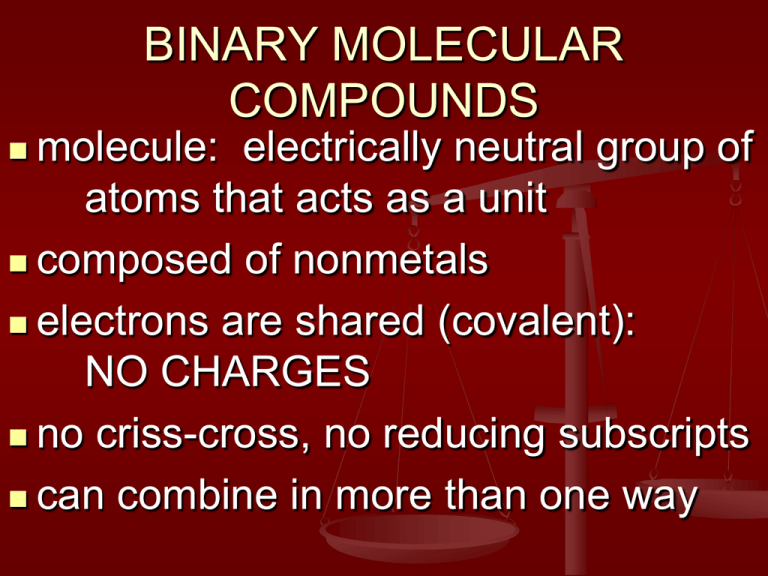
BINARY MOLECULAR COMPOUNDS molecule: electrically neutral group of atoms that acts as a unit composed of nonmetals electrons are shared (covalent): NO CHARGES no criss-cross, no reducing subscripts can combine in more than one way NAMING BINARY MOLECULES prefixes used to denote multiple atoms of one element in a molecule 1 2 3 4 5 mono- 6 di7 tri8 tetra- 9 penta- 10 hexaheptaoctanonadeca- NAMING BINARY MOLECULES element in name ends in –ide for oxides: if the prefix ends in a or o, LEAVE OUT the a or o monooxide becomes monoxide tetraoxide becomes tetroxide, etc. 2nd NAMING BINARY MOLECULES if there is ONE atom of the first element, omit the prefix mono– ex: CO carbon monoxide NOT monocarbon monooxide WRITING NAMES CO carbon monoxide CO2 carbon dioxide N2O dinitrogen monoxide Cl2O8 dichlorine octoxide WRITING NAMES OF2 oxygen difluoride N2O5 dinitrogen pentoxide P4O10 tetraphosphorus decoxide SO3 sulfur trioxide WRITING FORMULAS nitrogen trifluoride disulfur dichloride NF3 S2Cl2 dinitrogen tetroxide N2O4 carbon tetrachloride CCl4 DIATOMIC MOLECULES molecules with 2 atoms of the same element there are 7 – know them! N2 O2 H2 Cl2 I2 F2 Br2 or Br2 I2 N2 Cl2 H2 O2 F2 LEWIS DOT DIAGRAMS (ELECTRON DOT DIAGRAMS) show the valence electrons as dots inner electrons and atomic nuclei are represented by the symbol LEWIS DOT DIAGRAMS (ELECTRON DOT DIAGRAMS) 1A: 1 valence e group 2A: 2 valence e-, etc. group LEWIS DOT DIAGRAMS (ELECTRON DOT DIAGRAMS) diatomic molecules LEWIS STRUCTURES: STEPS 1. Count valence electrons and bonds using periodic table and octet rule – make a chart to get valence and shared electrons 2. Choose an arrangement of atoms (carbon in center, hydrogen or halogens on outside) LEWIS STRUCTURES: STEPS 3. Put in calculated number of bonds; single bonds first, then multiple bonds if necessary. 4. Where necessary to complete octets, put in non-bonding electrons in pairs. LEWIS STRUCTURES: STEPS 5. Check work by counting electrons in Lewis structure and comparing calculated number of valence electrons. LEWIS STRUCTURES CH2O C H H O V 4 1 1 6 12 lO S 4 H–C–H 1 1 2 8 / 2 = 4 bonds LEWIS STRUCTURES NH3 N H H H V 5 1 1 1 8 S H–N–H 3 H 1 1 1 6 / 2 = 3 bonds LEWIS STRUCTURES SO32– S O O O V 6 6 6 6 24 +2 26 S 2 2O–S–O 2 2 O 2 8 -2 6 / 2 = 3 bonds





