Experiment Three
advertisement
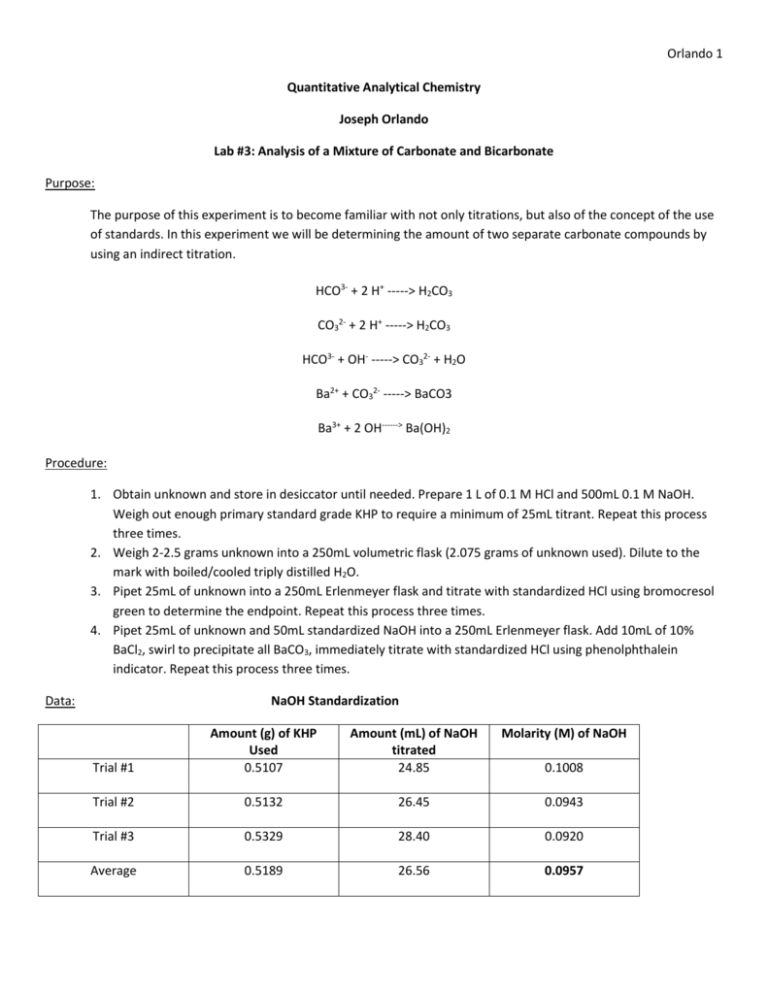
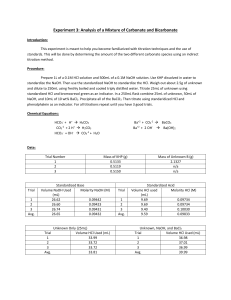
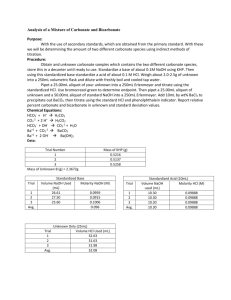
Orlando 1 Quantitative Analytical Chemistry Joseph Orlando Lab #3: Analysis of a Mixture of Carbonate and Bicarbonate Purpose: The purpose of this experiment is to become familiar with not only titrations, but also of the concept of the use of standards. In this experiment we will be determining the amount of two separate carbonate compounds by using an indirect titration. HCO3- + 2 H+ -----> H2CO3 CO32- + 2 H+ -----> H2CO3 HCO3- + OH- -----> CO32- + H2O Ba2+ + CO32- -----> BaCO3 Ba3+ + 2 OH------> Ba(OH)2 Procedure: 1. Obtain unknown and store in desiccator until needed. Prepare 1 L of 0.1 M HCl and 500mL 0.1 M NaOH. Weigh out enough primary standard grade KHP to require a minimum of 25mL titrant. Repeat this process three times. 2. Weigh 2-2.5 grams unknown into a 250mL volumetric flask (2.075 grams of unknown used). Dilute to the mark with boiled/cooled triply distilled H2O. 3. Pipet 25mL of unknown into a 250mL Erlenmeyer flask and titrate with standardized HCl using bromocresol green to determine the endpoint. Repeat this process three times. 4. Pipet 25mL of unknown and 50mL standardized NaOH into a 250mL Erlenmeyer flask. Add 10mL of 10% BaCl2, swirl to precipitate all BaCO3, immediately titrate with standardized HCl using phenolphthalein indicator. Repeat this process three times. Data: NaOH Standardization Amount (mL) of NaOH titrated 24.85 Molarity (M) of NaOH Trial #1 Amount (g) of KHP Used 0.5107 Trial #2 0.5132 26.45 0.0943 Trial #3 0.5329 28.40 0.0920 Average 0.5189 26.56 0.0957 0.1008 Orlando 2 HCl Standardization Amount (mL) of HCl titrated Molarity (M) of HCl Trial #1 Amount (mL) of NaOH used to determine HCl molarity 10.0 9.95 0.0962 Trial #2 10.0 9.80 0.0976 Trial #3 10.0 9.70 0.0986 Average 10.0 9.82 0.0975 Unknown Titration Molarity (M) of unknown sample Trial #1 Amount (mL) HCl titrated into unknown C sample 13.35 Trial #2 19.55 0.0762 Trial #3 20.40 0.0795 Average 17.77 0.0692 0.0521 BaCl2 Titration Trial #1 Amount (mL) of HCl titrated 26.73 Moles NaOH (initial) 0.00475 Moles NaOH (excess) 0.00261 Moles NaOH reacted 0.00214 Trial #2 25.85 0.00475 0.00252 0.00223 Trial #3 25.60 0.00475 0.00122 0.00353 Average 26.06 0.00475 0.00212 0.00263 Moles Carbonate Trial #1 Moles of Bicarbonate 0.00214 Trial #2 0.00047 Relative % Carbonate 1.36% Relative % Bicarbonate 6.29% 0.00223 0.00029 0.84% 6.56% Trial #3 0.00353 0.00031 0.89% 10.38% Average 0.00263 0.00036 1.03% 7.74% Orlando 3 Equations:. Mass (g) KHP Used in Step 1 0.025 𝐿 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 × 0.1 𝑚𝑜𝑙 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 1 𝑚𝑜𝑙 𝐾𝐻𝑃 204.22 𝑔 𝐾𝐻𝑃 × × = 0.51 𝑔 𝐾𝐻𝑃 1 𝐿 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 1 𝑚𝑜𝑙 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 1 𝑚𝑜𝑙 𝐾𝐻𝑃 Moles NaOH (initial) 𝑀 𝑁𝑎𝑂𝐻 × 𝐿 𝑁𝑎𝑂𝐻 𝑢𝑠𝑒𝑑 Moles NaOH (excess) 𝑀 𝐻𝐶𝑙 × 𝐿 𝐻𝐶𝑙 𝑢𝑠𝑒𝑑 Moles NaOH reacted 𝑖𝑛𝑖𝑡𝑖𝑎𝑙 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 − 𝑒𝑥𝑐𝑒𝑠𝑠 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 Moles Bicarbonate 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝑁𝑎𝑂𝐻 𝑟𝑒𝑎𝑐𝑡𝑒𝑑 × 1 𝑚𝑜𝑙𝑒 Bicarbonate 1 𝑚𝑜𝑙𝑒 𝑁𝑎𝑂𝐻 Moles Carbonate 𝑒𝑥𝑐𝑒𝑠𝑠 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝑁𝑎𝑂𝐻 − 𝑚𝑜𝑙𝑒𝑠 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 Relative % Carbonate 𝑚𝑜𝑙𝑒𝑠 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 × 60.007 𝑔 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 1 × × 100% 1 𝑚𝑜𝑙𝑒 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 2.075 𝑔 𝑈𝑛𝑘𝑛𝑜𝑤𝑛 𝐶 Relative % Bicarbonate 𝑚𝑜𝑙𝑒𝑠 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 × 61.014 𝑔 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 1 × × 100 1 𝑚𝑜𝑙𝑒 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 2.075 𝑔 𝑈𝑛𝑘𝑛𝑜𝑤𝑛 𝐶 Conclusion: In the titration to determine percent composition of carbonate and bicarbonate, it was calculated that the sample contained an average of 1.03% carbonate and 7.74% bicarbonate. This titration was accomplished by titrating standardized HCl (average molarity = 0.0975 M) into a solution of unknown and standardized NaOH (average molarity = 0.0957 M). The data obtained is relatively persistent between the three trials with a low calculated standard deviation. Orlando 4 Post Lab Questions: 1. What is meant by a primary standard? Primary standards are reagents which can be weighed easily and have a weight that is truly representative of the number of moles of substance contained. Primary standards are used in titration and are essential for determining an unknown concentration. 2. What is a secondary standard? A secondary standard is a compound used in analysis after evaluation against primary standards. 3. What is an indirect titration? An indirect titration is a two-step process. 1. Reactant X of unknown concentration is reacted with a reactant of known concentration (we will call this reactant B). 2. A titration is then performed to determine the amount of reactant B in excess. 4. Define titrant. A titrant is a solution of known concentration which is titrated into another solution to determine the concentration of another chemical compound.