Chapter 4 Worksheet
advertisement
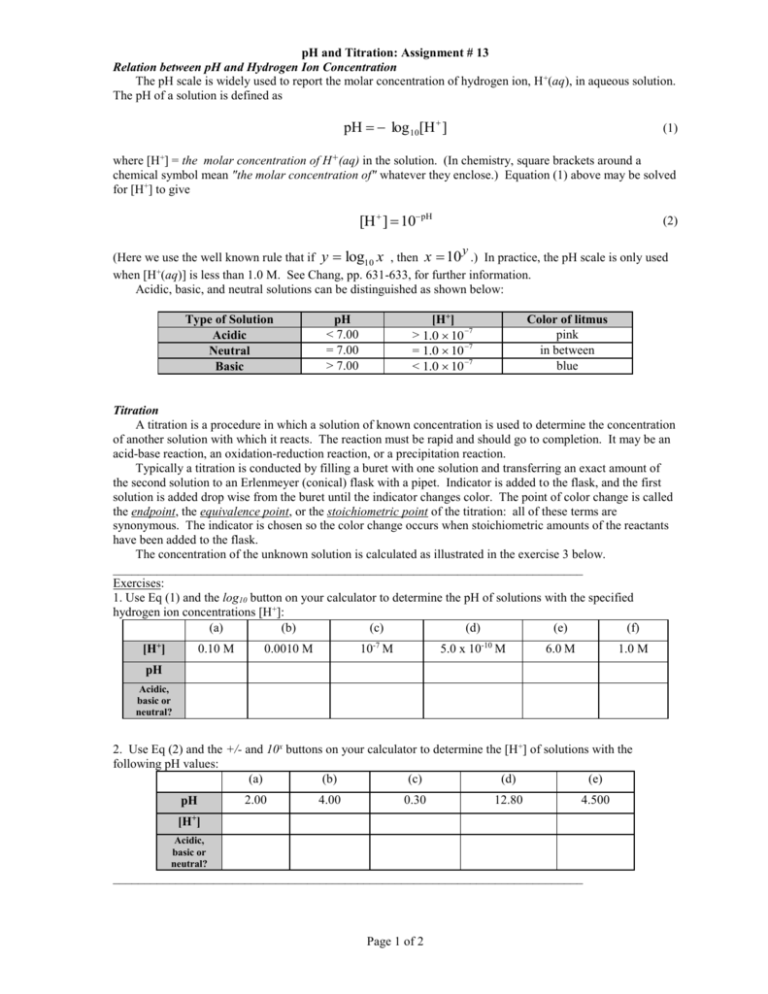
pH and Titration: Assignment # 13 Relation between pH and Hydrogen Ion Concentration The pH scale is widely used to report the molar concentration of hydrogen ion, H +(aq), in aqueous solution. The pH of a solution is defined as pH log 10[H ] (1) where [H+] = the molar concentration of H+(aq) in the solution. (In chemistry, square brackets around a chemical symbol mean "the molar concentration of" whatever they enclose.) Equation (1) above may be solved for [H+] to give [H ] 10pH (2) y (Here we use the well known rule that if y log10 x , then x 10 .) In practice, the pH scale is only used when [H+(aq)] is less than 1.0 M. See Chang, pp. 631-633, for further information. can be distinguished as shown below: Acidic, basic, and neutral solutions Type of Solution Acidic Neutral Basic [H+] > 1.0 10 7 = 1.0 10 7 < 1.0 10 7 pH < 7.00 = 7.00 > 7.00 Color of litmus pink in between blue Titration A titration is a procedure in which a solution of known concentration is used to determine the concentration of another solution with which it reacts. The reaction must be rapid and should go to completion. It may be an acid-base reaction, an oxidation-reduction reaction, or a precipitation reaction. Typically a titration is conducted by filling a buret with one solution and transferring an exact amount of the second solution to an Erlenmeyer (conical) flask with a pipet. Indicator is added to the flask, and the first solution is added drop wise from the buret until the indicator changes color. The point of color change is called the endpoint, the equivalence point, or the stoichiometric point of the titration: all of these terms are synonymous. The indicator is chosen so the color change occurs when stoichiometric amounts of the reactants have been added to the flask. The concentration of the unknown solution is calculated as illustrated in the exercise 3 below. ___________________________________________________________________________ Exercises: 1. Use Eq (1) and the log10 button on your calculator to determine the pH of solutions with the specified hydrogen ion concentrations [H+]: (a) (b) (c) (d) (e) (f) [H+] 0.10 M 10-7 M 0.0010 M 5.0 x 10-10 M 6.0 M 1.0 M pH Acidic, basic or neutral? 2. Use Eq (2) and the +/- and 10x buttons on your calculator to determine the [H+] of solutions with the following pH values: (a) (b) (c) (d) (e) pH 2.00 4.00 0.30 12.80 4.500 + [H ] Acidic, basic or neutral? ___________________________________________________________________________ Page 1 of 2 pH and Titration: Assignment # 13 3. A 25.00 mL sample of a 0.5250 M H2SO4 solution is titrated with a NaOH solution using phenolphthalein as the indicator. It is found that 22.07 mL of the NaOH solution is needed to reach the endpoint of the titration. What is the molarity of the NaOH solution? Notes: A good procedure is to divide the problem into the following steps: • Write the balanced overall equation for the reaction. • Determine the moles of the known solution available (in this case find the mol H2SO4 available). • Use your result from step 2 above and the stoichiometric ratio from the balanced equation to find the moles of the other solution (the one of unknown concentration) needed for complete reaction (in this case use the mol H2SO4 and your balanced acid-base equation to find the mol NaOH needed). (Alternatively, steps 2 and 3 can be combined into one step! Do you see how this could be accomplished?) • Obtain the molarity (M) by dividing the moles obtained in step 3 by the volume of the same solution used in the titration, and converting units to obtain mol/L (here begin by dividing mol NaOH by 22.07 mL). Page 2 of 2