Liq_sample_calcs
advertisement

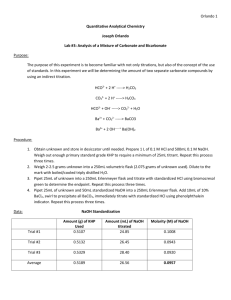
Sample Calculation **0.3mL of NaOH was required to standardized pure tap water. 0.3𝑚𝐿 𝑁𝑎𝑂𝐻 𝑉𝑒𝑞 = 𝑉𝑁𝑎𝑂𝐻 − 𝑉𝑠𝑎𝑚𝑝𝑙𝑒 ( ) 16𝑚𝐿 𝑃𝑢𝑟𝑒 𝐻2 𝑂 0.3𝑚𝐿 𝑁𝑎𝑂𝐻 𝑉𝑒𝑞 = 1.0𝑚𝐿 − 20𝑚𝐿 ∗ ( ) = 0.625𝑚𝐿 16𝑚𝐿 𝑃𝑢𝑟𝑒 𝐻2 𝑂 Where: 𝑉𝑒𝑞 is the equivalent volume (mL) of NaOH required to titrate the sample corrected for the naturally occurring acidity of pure tap water 𝑉𝑁𝑎𝑂𝐻 is the volume (mL)of NaOH used to titrate the sample read directly from the burette. 𝑉𝑠𝑎𝑚𝑝𝑙𝑒 is the volume (mL) of sample that was titrated. 𝑛𝑁𝑎𝑂𝐻 = 𝑀𝑁𝑎𝑂𝐻 ∗ 𝑉𝑒𝑞 𝑛𝑁𝑎𝑂𝐻 = 0.00095𝑀 ∗ 0.625𝑚𝐿 = 5.94 ∗ 10−7 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 𝑚𝐿 1000 𝐿 Where: 𝑛𝑁𝑎𝑂𝐻 is the moles NaOH required to titrate the sample corrected for the naturally occurring acidity of pure tap water 𝑀𝑁𝑎𝑂𝐻 is the Molarity (mols/L) of the NaOH used in the titration. 𝑉𝑒𝑞 is the equivalent volume (L) of NaOH required to titrate the sample corrected for the naturally occurring acidity of pure tap water 𝑛𝐴𝐴 = 𝑛𝑁𝑎𝑂𝐻 5.94 ∗ 10−7 𝑚𝑜𝑙𝑒𝑠 𝐴𝐴 = 5.94 ∗ 10−7 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 Where: 𝑛𝐴𝐴 is the moles of acetic acid in the titrated sample. 𝑛𝑁𝑎𝑂𝐻 is the moles NaOH required to titrate the sample corrected for the naturally occurring acidity of pure tap water 𝑀𝐴𝐴 = 𝑛𝐴𝐴 𝑉𝑠𝑎𝑚𝑝𝑙𝑒 𝑀𝐴𝐴 = 5.94 ∗ 10−7 𝑚𝑜𝑙𝑒𝑠 𝐴𝐴 = 2.97 ∗ 10−5 𝑀 20𝑚𝐿 𝑚𝐿 1000 𝐿 Where: 𝑀𝐴𝐴 is the Molarity (moles/L) of Acetic Acid in the sample. 𝑛𝐴𝐴 is the moles of acetic acid in the titrated sample. 𝑉𝑠𝑎𝑚𝑝𝑙𝑒 is the volume (mL) of sample that was titrated. 𝑋𝐴𝐴 = 𝑋𝐴𝐴 = 𝑛𝐴𝐴 𝑉𝑠𝑎𝑚𝑙𝑒 ∗ 𝜌 𝑀𝑊 5.94 ∗ 10−7 𝑚𝑜𝑙𝑒𝑠 𝐴𝐴 = 5.35 ∗ 10−7 20𝑚𝐿 ∗ 1.0𝑔/𝑚𝐿 18𝑔/𝑚𝑜𝑙 Where: 𝑋𝐴𝐴 is the mole fraction of Acetic Acid in the sample. 𝑛𝐴𝐴 is the moles of acetic acid in the titrated sample. 𝑉𝑠𝑎𝑚𝑝𝑙𝑒 is the volume (mL) of sample that was titrated. 𝜌 is the density (g/mL) of the sample. For water samples assume that the density is that of pure water (1.0g/mL), and for the oil samples assume the density is that of pure oil (0.853g/mL). 𝑀𝑊 is the molecular weight of the sample. For the water samples assume the weight is 18g/mol and for the oil samples assume the weight is __________________. 𝑊= 𝑊= 𝑀𝑊𝐴𝐴 ∗ 𝑀𝐴𝐴 ∗ 100 𝜌 60.05𝑔/𝑚𝑜𝑙 ∗ 2.97 ∗ 10−5 𝑀 ∗ 100 = 1.78 ∗ 10−4 % 1.0𝑔/𝑚𝐿 Where: W is the weight percent of Acetic Acid in the sample. ρ is the density (g/mL) of the sample. For water samples assume that the density is that of pure water (1.0g/mL), and for the oil samples assume the density is that of pure oil (0.853g/mL). 𝑀𝐴𝐴 is the Molarity (moles/mL) of Acetic Acid in the sample.