Analysis of a Mixture of Carbonate and Bicarbonate
advertisement
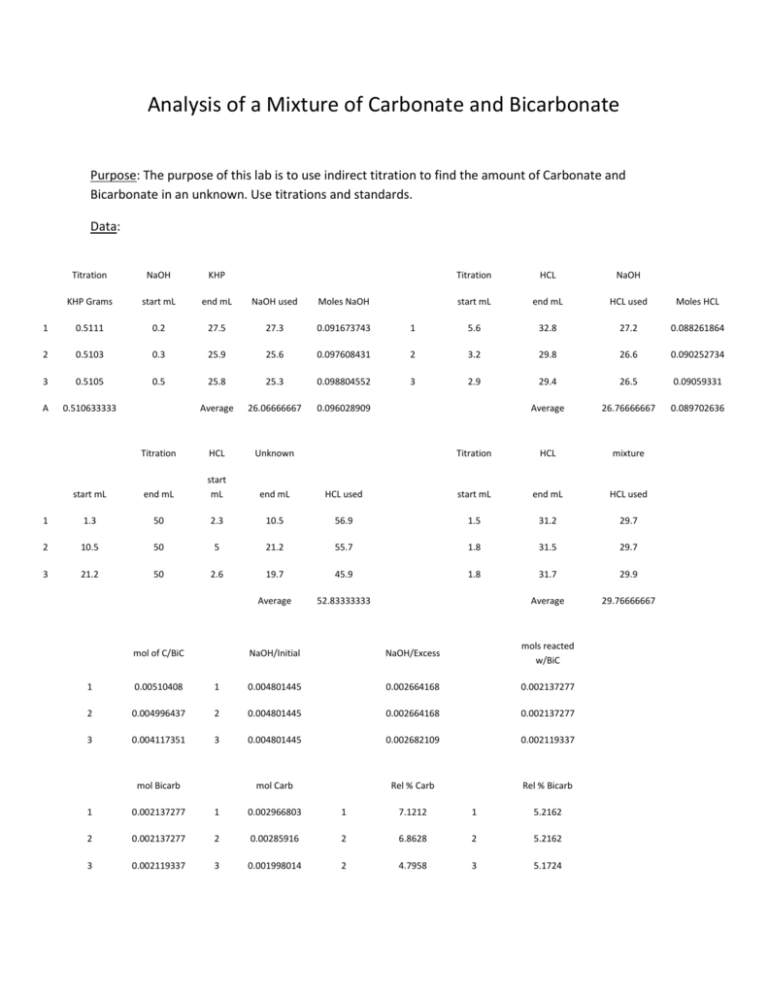
Analysis of a Mixture of Carbonate and Bicarbonate Purpose: The purpose of this lab is to use indirect titration to find the amount of Carbonate and Bicarbonate in an unknown. Use titrations and standards. Data: Titration NaOH KHP Titration HCL NaOH KHP Grams start mL end mL NaOH used Moles NaOH start mL end mL HCL used Moles HCL 1 0.5111 0.2 27.5 27.3 0.091673743 1 5.6 32.8 27.2 0.088261864 2 0.5103 0.3 25.9 25.6 0.097608431 2 3.2 29.8 26.6 0.090252734 3 0.5105 0.5 25.8 25.3 0.098804552 3 2.9 29.4 26.5 0.09059331 A 0.510633333 Average 26.06666667 0.096028909 Average 26.76666667 0.089702636 Titration HCL Unknown Titration HCL mixture start mL end mL start mL end mL HCL used start mL end mL HCL used 1 1.3 50 2.3 10.5 56.9 1.5 31.2 29.7 2 10.5 50 5 21.2 55.7 1.8 31.5 29.7 3 21.2 50 2.6 19.7 45.9 1.8 31.7 29.9 Average 52.83333333 Average 29.76666667 mol of C/BiC NaOH/Initial NaOH/Excess mols reacted w/BiC 1 0.00510408 1 0.004801445 0.002664168 0.002137277 2 0.004996437 2 0.004801445 0.002664168 0.002137277 3 0.004117351 3 0.004801445 0.002682109 0.002119337 mol Carb Rel % Carb Rel % Bicarb mol Bicarb 1 0.002137277 1 0.002966803 1 7.1212 1 5.2162 2 0.002137277 2 0.00285916 2 6.8628 2 5.2162 3 0.002119337 3 0.001998014 2 4.7958 3 5.1724 Aev. 6.2599 Aev. 5.2016 Stand Dev: 1.27453 Stand Dev: 0.02528 Calculations: . 025 𝐿 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 × . 1 𝑚𝑜𝑙 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 1 𝑚𝑜𝑙 𝐾𝐻𝑃 204.22 𝑔 𝐾𝐻𝑃 × × = 0.51 𝑔 𝐾𝐻𝑃 1 𝐿 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 1 𝑚𝑜𝑙 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 1 𝑚𝑜𝑙 𝐾𝐻𝑃 0.1 𝑀 𝑁𝑎𝑂𝐻 × 0.5111 𝑔 𝐾𝐻𝑃 × 𝑀 𝐻𝐶𝑙 = 39.995 𝑔 𝑁𝑎𝑂𝐻 × 0.5 𝐿 ≅ 2 𝑔 𝑁𝑎𝑂𝐻 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 1 𝑚𝑜𝑙 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 1 × × = .0917𝑀 𝑁𝑎𝑂𝐻 204.22 𝑔 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝐾𝐻𝑃 . 0273 𝐿 𝑁𝑎𝑂𝐻 (𝑀 𝑁𝑎𝑂𝐻)(𝑉𝑜𝑙. 𝑁𝑎𝑂𝐻) (. 0917 𝑀)(25.0 𝑚𝐿) = = .0883 𝑀 𝐻𝐶𝑙 𝑉𝑜𝑙. 𝐻𝐶𝑙 27.2 𝑚𝐿 . 0897𝑀 𝐻𝐶𝑙 × .0569 𝐿 𝐻𝐶𝑙 (𝑏𝑟𝑜𝑚𝑜𝑐𝑟𝑒𝑠𝑜𝑙) = 0.0051 𝑚𝑜𝑙 . 0960 𝑀 𝑁𝑎𝑂𝐻 × .0500 𝐿 𝑁𝑎𝑂𝐻 (𝑎𝑑𝑑𝑒𝑑) = 0.0048 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 (𝑖𝑛𝑖𝑡𝑖𝑎𝑙) 0.0897 𝑀 𝐻𝐶𝑙 × .0297 𝐿 𝐻𝐶𝑙 (𝑝ℎ𝑒𝑛𝑎𝑙𝑝ℎ𝑡ℎ𝑎𝑙𝑒𝑖𝑛) × 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 = 0.00266 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 (𝑒𝑥𝑐𝑒𝑠𝑠) 1 𝑚𝑜𝑙 𝐻𝐶𝑙 [. 0048 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 (𝑖𝑛𝑖𝑡𝑖𝑎𝑙)] − [. 00266 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 (𝑒𝑥𝑐𝑒𝑠𝑠)] = 0.00214 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 (𝑟𝑒𝑎𝑐𝑡𝑒𝑑) 0.00214 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 × 1 𝑚𝑜𝑙 Bicarbonate = 0.00214 𝑚𝑜𝑙 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 [. 0051 𝑚𝑜𝑙 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 & 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒] − [. 00214 𝑚𝑜𝑙 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒] = 0.00296𝑚𝑜𝑙 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 . 00296 𝑚𝑜𝑙 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 × . 00214 𝑚𝑜𝑙 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 × 60.007 𝑔 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 1 × × 100 = 7.12% 1 𝑚𝑜𝑙 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 2.50 𝑔 𝑈𝑛𝑘𝑛𝑜𝑤𝑛 61.014 𝑔 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 1 × × 100 = 5.22% 1 𝑚𝑜𝑙 𝐵𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 2.50 𝑔 𝑈𝑛𝑘𝑛𝑜𝑤𝑛 (7.1212 − 6.2599)2 + (6.8628 − 6.2599)2 + (4.7958 − 6.2599)2 √ = 1.27453 2 Conclusions: In the 2.5 gram sample of unknown A, that my partner and I massed; .178 grams were Carbonate and .1305 grams were Bicarbonate. Any error would be due to human error during the titrations, and or errors in the calculations. Primary Standards are not standardized by using other standards and are used to calculate secondary standard. A secondary standard is a solution at has be standardized using a primary standard. A titrant is a solution that has a known concentration, which is added to another solution to determine its concentration. Indirect titration( also called back titration) is finding the concentration of something by looking at how much reagent was need to titrate a second reagent, while knowing the concentration of the first reagent.





















