Analysis of a Mixture of Carbonate and Bicarbonate
advertisement
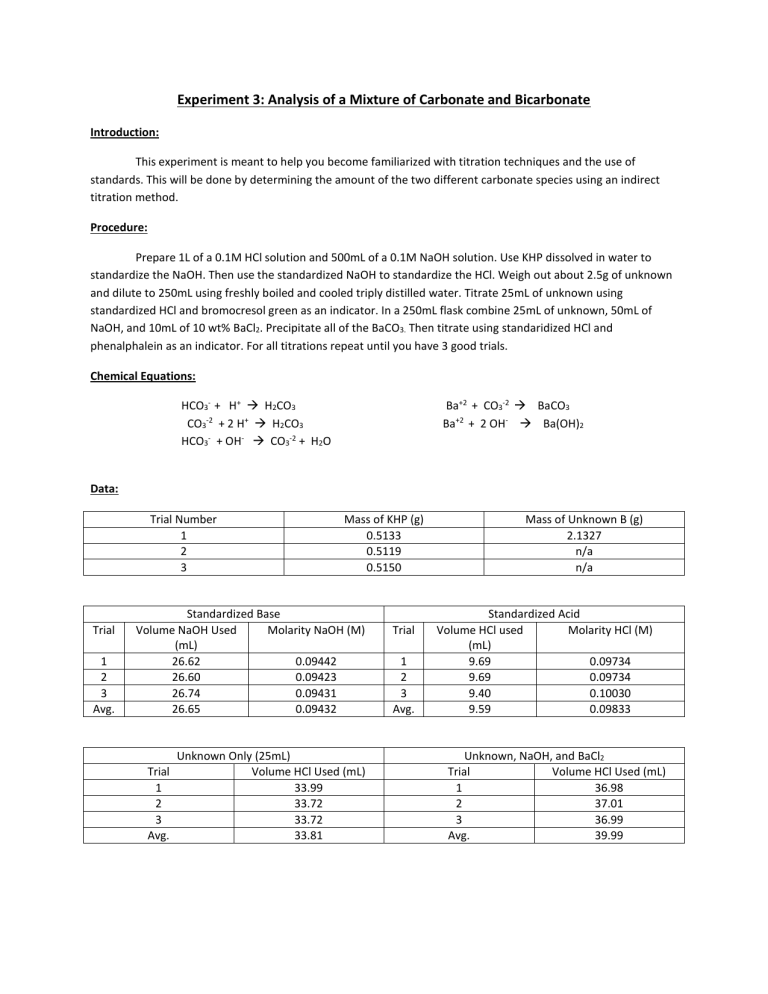
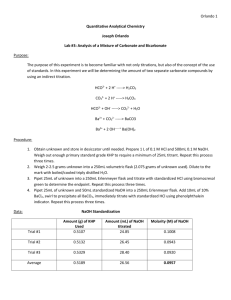
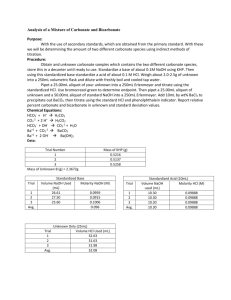
Experiment 3: Analysis of a Mixture of Carbonate and Bicarbonate Introduction: This experiment is meant to help you become familiarized with titration techniques and the use of standards. This will be done by determining the amount of the two different carbonate species using an indirect titration method. Procedure: Prepare 1L of a 0.1M HCl solution and 500mL of a 0.1M NaOH solution. Use KHP dissolved in water to standardize the NaOH. Then use the standardized NaOH to standardize the HCl. Weigh out about 2.5g of unknown and dilute to 250mL using freshly boiled and cooled triply distilled water. Titrate 25mL of unknown using standardized HCl and bromocresol green as an indicator. In a 250mL flask combine 25mL of unknown, 50mL of NaOH, and 10mL of 10 wt% BaCl2. Precipitate all of the BaCO3. Then titrate using standaridized HCl and phenalphalein as an indicator. For all titrations repeat until you have 3 good trials. Chemical Equations: HCO3- + H+ H2CO3 CO3-2 + 2 H+ H2CO3 HCO3- + OH- CO3-2 + H2O Ba+2 + CO3-2 BaCO3 Ba+2 + 2 OH- Ba(OH)2 Data: Trial Number 1 2 3 Trial 1 2 3 Avg. Mass of KHP (g) 0.5133 0.5119 0.5150 Standardized Base Volume NaOH Used Molarity NaOH (M) (mL) 26.62 0.09442 26.60 0.09423 26.74 0.09431 26.65 0.09432 Trial 1 2 3 Avg. Unknown Only (25mL) Volume HCl Used (mL) 33.99 33.72 33.72 33.81 Trial 1 2 3 Avg. Mass of Unknown B (g) 2.1327 n/a n/a Standardized Acid Volume HCl used Molarity HCl (M) (mL) 9.69 0.09734 9.69 0.09734 9.40 0.10030 9.59 0.09833 Unknown, NaOH, and BaCl2 Trial Volume HCl Used (mL) 1 36.98 2 37.01 3 36.99 Avg. 39.99 Calculated Data Trial Mol CO3&HCO3 1 0.003342 2 0.003316 3 0.003316 Avg Std Dev Mol NaOHtotal 0.004716 0.004716 0.004716 Mol NaOHw/HCl 0.003636 0.003639 0.003637 Mol NaOHw/HCO3 0.00108 0.001077 0.001079 Mol CO3 Mass% HCO3 Mass% CO3 0.002262 0.002239 0.002237 30.90 30.81 30.87 30.86 0.082765 63.65 63.0 62.94 63.20 0.039383 Calculations: Volume used to titrate: ∆𝑉 = 𝑉𝑓𝑖𝑛𝑎𝑙 − 𝑉𝑖𝑛𝑖𝑡𝑖𝑎𝑙 Molarity of NaOH: 𝑀𝑁𝑎𝑂𝐻 = (𝑚𝐾𝐻𝑃 ∗ 1 𝑚𝑜𝑙 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 ∗ ) ÷ ∆𝑉 204.22𝑔 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝐾𝐻𝑃 Molarity of HCl: ̅𝑁𝑎𝑂𝐻 𝑉𝑁𝑎𝑂𝐻 = 𝑀𝐻𝐶𝑙 ∆𝑉𝐻𝐶𝑙 𝑀 Moles of Carbonate and Bicarbonate: ̅𝐻𝐶𝑙 ∗ 𝑉𝐻𝐶𝑙 𝑎𝑑𝑑𝑒𝑑 = 𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑙 𝑀 𝑚𝑜𝑙𝑒𝑠 𝑎𝑐𝑖𝑑 = 𝑚𝑜𝑙𝑒𝑠 𝑏𝑎𝑠𝑒 Total moles of NaOH: ̅𝑁𝑎𝑂𝐻 ∗ 𝑉𝑁𝑎𝑂𝐻 𝑖𝑛 𝑠𝑎𝑚𝑝𝑙𝑒 = 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 𝑀 Moles of NaOH reacted with HCl: ̅𝐻𝐶𝑙 ∗ 𝑉𝐻𝐶𝑙 𝑎𝑑𝑑𝑒𝑑 = 𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑙 𝑀 𝑚𝑜𝑙𝑒𝑠 𝑎𝑐𝑖𝑑 = 𝑚𝑜𝑙𝑒𝑠 𝑏𝑎𝑠𝑒 Moles NaOH reacted with Bicarbonate: 𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 𝑤𝑖𝑡ℎ 𝐻𝐶𝑂3 = 𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 𝑡𝑜𝑡𝑎𝑙 − 𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 𝑤𝑖𝑡ℎ 𝐻𝐶𝑙 Moles of Bicarbonate: 𝑚𝑜𝑙𝑒𝑠𝐻𝐶𝑂3 = 𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 Moles of Carbonate: 𝑚𝑜𝑙𝑒𝑠𝐶𝑂3 = 𝑚𝑜𝑙𝑒𝑠𝐶𝑂3&𝐻𝐶𝑂3 − 𝑚𝑜𝑙𝑒𝑠𝐻𝐶𝑂3 Mass percent of Bicarbonate: 61.016𝑔 𝐻𝐶𝑂3 𝑚𝑜𝑙𝑒𝑠𝐻𝐶𝑂3 ∗ = 𝑚 𝐻𝐶𝑂3 1 𝑚𝑜𝑙 𝐻𝐶𝑂3 𝑚 𝐻𝐶𝑂3 ∗ 100% = 𝑚𝑎𝑠𝑠% 𝑚 𝑠𝑎𝑚𝑝𝑙𝑒/10 Mass percent of Carbonate: 60.008𝑔 𝐶𝑂3 𝑚𝑜𝑙𝑒𝑠𝐶𝑂3 ∗ = 𝑚 𝐶𝑂3 1 𝑚𝑜𝑙 𝐶𝑂3 𝑚 𝐶𝑂3 ∗ 100% = 𝑚𝑎𝑠𝑠% 𝑚 𝑠𝑎𝑚𝑝𝑙𝑒 Example for trial 1: 26.62𝑚𝐿 𝑁𝑎𝑂𝐻 = 28.61𝑚𝐿 − 1.99𝑚𝐿 Molarity of NaOH for trial 1: 0.09442𝑀 = (0.5133𝑔 ∗ 1 𝑚𝑜𝑙 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝑁𝑎𝑂𝐻 ∗ ) ÷ .02662𝐿 204.22𝑔 𝐾𝐻𝑃 1 𝑚𝑜𝑙 𝐾𝐻𝑃 Molarity of HCl for trial 1: 0.09432𝑀(10𝑚𝐿) = 𝑀𝐻𝐶𝑙 (9.69𝑚𝐿) 𝑀𝐻𝐶𝑙 = 0.09734 Moles of Carbonate and Bicarbonate for trial 1: 0.09833𝑀 ∗ 0.03399𝐿 = 0.003342𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑙 = 0.003342𝑚𝑜𝑙𝑒𝑠 𝑐𝑎𝑟𝑏./𝑏𝑖𝑐𝑎𝑟𝑏. Total moles of NaOH for trial 1: 0.09432𝑀 ∗ 0.0500𝐿 = 0.004716 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 Moles of NaOH reacted with HCl for trial 1: 0.09833𝑀 ∗ 0.03698𝐿 = 0.003636𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑙 = 0.003636𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 𝑤𝑖𝑡ℎ 𝐻𝐶𝑙 Moles NaOH reacted with Bicarbonate for trial 1: 𝑚𝑜𝑙𝑤/𝐻𝐶𝑂3 = 0.004716𝑚𝑜𝑙𝑡𝑜𝑡𝑎𝑙 − 0.003636𝑚𝑜𝑙𝑤/𝐻𝐶𝑙 = 0.00108 𝑚𝑜𝑙𝑒𝑠𝑁𝑎𝑂𝐻 𝑤𝑖𝑡ℎ 𝐻𝐶𝑂3 Moles of Bicarbonate for trial 1: = 0.00108 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 Moles of Carbonate for trial 1: 𝑚𝑜𝑙𝐶𝑂3 = 0.003342𝑚𝑜𝑙𝐶𝑂3&𝐻𝐶𝑂3 − 0.00108𝑚𝑜𝑙𝐻𝐶𝑂3 = 0.002262𝑚𝑜𝑙𝑒𝑠 𝐶𝑂3 Mass percent of Bicarbonate for trial 1: 0.00108𝑚𝑜𝑙𝐻𝐶𝑂3 ∗ 61.016𝑔 𝐻𝐶𝑂3 = 0.065897𝑚 𝐻𝐶𝑂3 1 𝑚𝑜𝑙 𝐻𝐶𝑂3 0.065897𝑔 𝐻𝐶𝑂3 ∗ 100% = 30.90% 2.1327𝑔/10 Mass percent of Carbonate for trial 1: 60.008𝑔 𝐶𝑂3 0.002262𝑚𝑜𝑙𝐶𝑂3 ∗ = 0.135738𝑔 𝐶𝑂3 1 𝑚𝑜𝑙 𝐶𝑂3 0.135738𝑔 𝐶𝑂3 ∗ 100% = 63.65% 2.1327𝑔/10 Discussion: A primary standard is a reagent that is pure and stable enough to be used immediately after weighing. Then a secondary standard is then standardized in the lab using the primary standard. This means it is calculated to contain a specific amount of analyte. An indirect titration also known as a back titration involves determining the concentration of analyte through reacting it with a known amount of moles of excess reagent. Excess is titrated with a second reagent and concentration of analyte is related to the amount of reagent consumed. The titrant is the solution in the burette during a titration and is used to determine the concentration of the unknown solution. Conclusion: The calculated standard deviations are acceptable considering they are less than 1 percent for the mass percentages. The data and calculations for the three trials for this experiment resulted in consistent results for the mass percent of both bicarbonate and carbonate. The resulting mass percentages for the unknown were an average of 30.86% of bicarbonate and an average of 63.20% of carbonate. This method proves to be successful.