HYDROCARBONS
advertisement
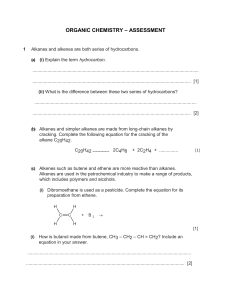
HYDROCARBONS Hydrocarbons Compounds that contain only carbon atoms and hydrogen atoms. Obtained from crude oil by fractional distillation and can be classified into different groups. There are different types of hydrocarbons. Each type is known as a homologous series. Members of each series share similar structures and properties. Homologous series – Alkanes (single covalent bonds) Homologous series - Alkenes (contain one C=C double covalent bond) Properties of Alkanes Alkanes are called saturated hydrocarbons because they only have single bonds between the carbon atoms. C2 H6 (graphical formula) Molecular formula The name of the alkane depends upon how many carbon atoms the chain has. 1C methane 2C ethane 3C propane 4C butane The chain may have a branch. The name of the alkane will indicate this. 2-methyl propane Properties of Alkanes The general formula for an alkane is: CnH2n+2 As the number of carbon atoms in a chain increases, bpt and viscosity increases. This is because the molecules are attracted to each other and as chain length increases so does the attraction (more energy needed to separate them). (Flammability decreases). Properties of Alkanes Alkanes burn in a plentiful supply of air to release energy (this is why they are used as fuels). Burning (properly called combustion) also produces carbon dioxide and water vapour. COMPLETE COMBUSTION of methane CH4 (g) + O2 (g) → CO2 (g) + H2O (g) Can you balance the equation??? Blue flame (COMPLETE COMBUSTION) If the oxygen supply is restricted then the combustion of alkanes produces the poisonous gas carbon monoxide and carbon (soot – responsible for the blackening of buildings). INCOMPLETE COMBUSTION of methane CH4 (g) + O2 (g) → CO (g) + H2O (g) Can you balance the equation??? Orange flame (INCOMPLETE COMBUSTION) Alkenes Alkenes are also hydrocarbons but they contain a double bond between two of the carbon atoms. Alkenes are said to be unsaturated because of the double bond General formula: CnH2n Properties of alkenes Bpt increases with chain length. Alkenes undergo combustion (burning) in a similar way to alkanes. The double bond makes them more reactive than alkanes, therefore undergo more chemical reactions Bromine can be added to the double bond. This is called an addition reaction. The reaction makes the red colour of 'bromine solution' disappear. (This reaction is used to test for a double bond a.k.a unsaturated molecule). Testing for unsaturation (g) + Ethene (colourless) Br2 (l) (aq) (orange) (colourless) http://www.youtube.com/watch?v=2C_6ax 2TsV8 Questions to do: Pg 175-177 Q3, 7, 13 Pg 256 Q21, 22