Summary of Organic chemistry
advertisement

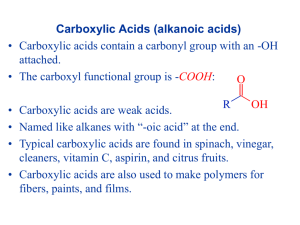
Organic chemistry Summary of Organic Compounds Class Alkanes General Formula CnH2n+2 Alkenes CnH2n Alkynes CnH2n-2 Aromatic Physical Properties/Features <C5 gases; C5-C15 liquid; >C15 solids. Insoluble in H2O . -methane main component of natural gas, higher alkanes form crude oil and petroleum distillates. -physical properties similar to alkanes -product of catalytic cracking -ethenepolyethylene (plastic bags) -propene propylene (clothing) -physical properties similar to alkanes/alkenes -acetylene (ethyne) is only important commercial alkyne -distinct (often pleasant) odours; hence aromatic -from distillation of coal (benzene, toluene etc) -from crude oil (cyclization and hydrogenation) Haloalkane (X=F,Cl,Br,I) R-Xn Amines R-NH2 Nitroalkane R-NO2 -low solubility in water -somewhat pleasant odours Alcohol R-OH -ethanol form fermentation of grains etc. -solubility in H2O inverse to chain length -poisonous Aldehyde -slightly soluble in water (solubility decreases with chain length) O Ketone R C H O ether R C R' R O R' Carboxylic acid -CH3Cl refrigerant -CH2Cl2 propellant, solvent (caffeine extractor from coffee) -fishy, ammoniacal smell -natural flavouring/odour in some cheeses -somewhat soluble in water (solubility decreases with chain length) O R C OH Esters O R C O Amide O R C NH2 R' -Insoluble in water Good solvent for other organic cpnds Ethoxyethane (diethyl ether) used as anaesthetic -ethanoic (acetic) acid produced by fermentation of fruit sugar ethanol ethanoic acid (enzyme req'd) -synthesized from ethyne (acetylene) - most esters have pleasant, fruity flavours -natural and synthetic flavouring in flowers, fruit and candy -natural waxes=esters of long chain acids and alcohol -normally insoluble in water Chemical Properties non-reactive compared with other organic cpnds. r’n types: combustion, cracking and substitution same rxs as alkanes plus addition r’n at double bonds and polymerization same as alkenes similar to alkanes -ring structure unreactive -substitution of H'(s) on the ring -CFC'(s) ozone depletants -CCl4 carcinogenic -substitution r’ns -organic base R-NH2 + H2O R-NH3 + OH-react with organic acids to form amides -unreactive at room temp. -explosives (TNT=trinitrotoluene, nitroglycerine "gun-cotton") -synthesized by hydration of alkenes with H+ catalyst; C2H4 + H2O C2H5OH -synthesis of esters by rx with carboxylic acids -readily oxidizes to carboxylic acid 2R-CHO + O2 2RCOOH relatively unreactive Highly flammable (unstable -obond) -typical acid reactions R-COOH + H2O R-COO- + H3O+ readily synthesized by r’n of alcohol +carboxylic acid in presence of catalyst (H2SO4) -very weak bases involving NH2 -form strong bonds connecting amino acids in proteins (peptide bonds) -strong bonds in "nylon"