Alkene - TangHua2012-2013
advertisement

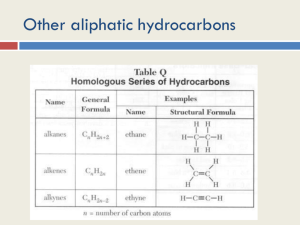
Chemistry Teachers Group Members Annie Ma George Shao David Wu Topic Alkene & Alkyne INTRODUCTION Hydrocarbons consist of alkanes, alkenes, and alkynes. Alkanes are single-bond, while the other two are multi-bonded. (Double-bond alkene, triple-bond alkyne) Alkanes are saturated hydrocarbons, while the other two are unsaturated ones. MATERIAL 1) PPT 2) VIDEO 3) POSTER 4) EXPERIMENT EXPLANATION OF MATERIAL Alkene Definition: Organic compound containing carbon-carbon double bonds Rules for Naming • Rules are similar as the naming of alkane • “ane” “ene” • A Number Lower numbered C In front of parent hydrocarbon, separated by ”–” Condensed Structure Ex. 2-hexene • “hex”= 6 C ( C C C C C C ) • “ene”= C=C bond • 2 = bonds C#2~#3 ( C-C=C-C-C-C ) • C = 4 bonds • Count bonds between each C and its neighbours • Subtract that # from 4 • ( =# of H attached to each C) • (#1=4-1=3, #2=4-3=1) • CH3-CH=CH-CH2-CH2-CH3 Geometry • Angles are approximately 120 degrees • 3 atoms connected to each C lie flat • Rigid (stronger) Physical properties • Comparable with alkanes • Acidity levels of alkenes are much higher than the ones in alkanes • States depends on molecular mass • The simplest alkenes, ethene, propene and butene are gases. Chemical properties • Relatively stable • More reactive than alkanes due to the presence of a carbon–carbon pi-bond Alkyne Definition: Organic compound containing carbon-carbon triple bonds Rules for Naming • “ene” “yne” Condensed Structure • The same as that of alkenes. Geometry • In acetylene, the H–C≡C bond angles are 180° • Cyclic alkynes are rare. • Benzyne is highly unstable. • The C≡C bond distance (121 pm) is much shorter than the C=C distance in alkenes (134 pm) or the C-C bond in alkanes (153 pm). Physical properties • Insoluble in water, but are fairly soluble in organic solvents • The melting and boiling points of alkynes increase with molecular mass. Chemical properties • Alkynes are characteristically more unsaturated than alkenes. • Alkynes are usually more reactive than alkenes.