Organic Nomenclature * The Basics
advertisement
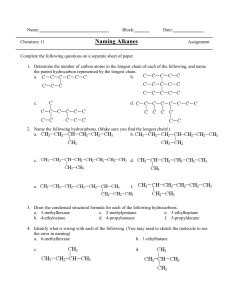
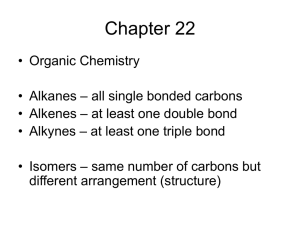
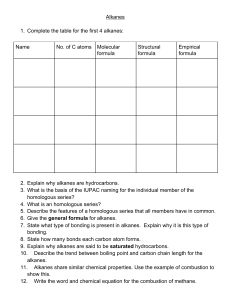
Organic Nomenclature – The Basics Organic Nomenclature • Naming compounds that contain Carbon and Hydrogen • Hydrocarbons: Contain only H and C Alkanes • • • • Saturated Hydrocarbons (Max. # of H) Only single bonds CnH2n+2 for chains CnH2n for cyclic structures Naming Unbranched Alkanes • Determined by # of Carbons, end in -ane 1: meth2: eth3: prop4: butC6H14 : Hexane CH4 : ? 5: pent6: hex7: sept8: oct 9: non10: dec- C3H8 : Propane C4H10 : ? Branched Alkanes • Alkyl Group: branch with ONLY H and C • Methyl: 1 C: -CH3 • Ethyl: 2 C: -CH2CH3 • Propyl: 3 C: -CH2CH2CH3 Naming Alkanes 1) Find longest C chain containing the most substituents, count C, write name 2) Number C from closest substituent 3) Name substituents in alpha order -More than one of each type use di-, tri- prefixes 4) Indicate positions of substiuents 5) Add commas between #s and hyphens between # & letter Alkenes (pg. 79) • • • • • Hydrocarbons with at least 1 double bond CnH2n Unsaturated Carbons Prefixes are same as alkanes, ending –ene C2H4 : ethene C4H8 : butene Alkynes • • • • • Hydrocarbons with at least 1 triple bond CnH2n-2 Unsaturated Carbons Prefixes are same as alkanes, ending –yne C3H4 : propyne C4H6 : butyne Naming Alkenes & Alkynes • Follow Alkane Rules • Looking for longest chain that contains double bond (-ene) or triple (–yne) • Multiple double or triple bonds add prefix (di-, tri-) and location before ending – Ex: Prop-1,2-diene or 1,2-propdiene