summary notes - Kinross High School
advertisement

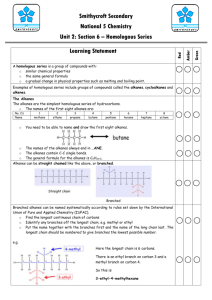
Nature’s Chemistry – Summary File Given below are some of the basics of what you will need to know for this topic. In order to make sure that you have revised all aspects of the unit, you also will have to look at your noted booklet. Fuels: A fuel is a substance which combusts to give out energy. In order for combustion to happen, all three aspects of the fire triangle need to be present. In combustion, the fuel burns with oxygen to form oxides, e.g. CH4 + 2O2 -> CO2 + 2H20 Fossil fuels are coal, crude oil, natural gas and peat. These fuels are made by dead plants (coal and peat) or dead sea animals (oil and gas) which are compressed and heated in layers for millions of years. Fossil fuels are finite (will run out), non-renewable fuels and combust to give out gases which causes global warming. Fossil fuels are hydrocarbons (i.e. contain only carbon and hydrogen) All hydrocarbons combust to give carbon dioxide and water. This can be proved as carbon dioxide will turn lime water milky and water is a colourless liquid that boils at 100⁰C and freezes at 0⁰C. The apparatus to prove this would look like: Complete combustion happens when there is an ample supply of oxygen, however, when not enough oxygen is present you get incomplete combustion. When a hydrocarbon is combusted incompletely, carbon (soot) or carbon monoxide can be made as well as water. dangerous as carbon monoxide is a toxic gas. This is Air pollution is often caused by combustion. An example is acid rain which is caused by SO2 and NOx gases. SO2 is caused by burning fossil fuels which contain sulphur as an impurity and NOx gases are caused by sparks in car engines and by lightning. Both these gases dissolve in water to form acids. Acid rain corrodes buildings and bridges, kills trees and causes aluminium to leech into waterways, killing fish. Air pollution can be decreased in a number of ways. A catalytic converter containing platinum, palladium or rhodium in a honeycomb structure can change harmful exhaust gases into harmless gases. A lean burn engine can be used in cars to ensure full combustion. Scrubbers can be used in power station chimneys to remove sulphur dioxide. Unleaded or low sulphur petrols can be used. Fossil fuels are described as non-renewable, meaning that they cannot be replaced. However, increasingly electricity is being made from renewable sources (wind, hydro, biofuels) which will never run out. The carbon cycle show how carbon is changed from one form to another: The greenhouse effect explains why the world is steadily warming due to gases like CO2 being released into the environment. The effects of global warming (greenhouse effect) include icecaps melting, more severe weather, habitat destruction and flooding. We can reduce this by burning less fossil fuels and thus reducing our carbon footprint. Using carbon capture technologies may also help. Distillation is a technique that separates liquids with different boiling points. Fractional distillation is a way of purifying crude oil, to produce groups of hydrocarbons (fractions) that all have similar boiling points and chain lengths. As the chain length gets longer, the fractions become more viscous (more difficult to pour), less flammable and have higher melting and boiling points. Hydrocarbons: A hydrocarbon is a compound made up of only hydrogen and carbon. Hydrocarbons are bonded covalently by sharing electrons. A homologous series is a family of hydrocarbons that have a general formula, have similar chemical properties and show a regular increase in physical properties. The simplest homologous series is the alkanes: The shortened structural formula can also be used to save time, e.g. ethane = CH3-CH3 All alkanes are saturated (contain only single bonds) and are based on the tetrahedral shape: The alkenes are a second homologous series. They all have a double bond in their structure. This means that they are unsaturated. Hydrocarbons can be cracked to produce shorter, more valuable and unsaturated compounds. An aluminium oxide or iron catalyst is used in the apparatus shown below: Unsaturation can be tested for by adding bromine water. If you add bromine water to a compound with a double or triple bond it will quickly decolourise. This allows us to tell the difference between alkanes and alkenes. One product of cracking will always be unsaturated and the products will always be smaller in chain length. However, the total number of carbons and hydrogens in the products is always equal to the number of carbons and hydrogens in the reactant. Homologous Series: The cycloalkanes are another homologous series of hydrocarbons. They are saturated and are connected in a circle. All homologous series have a general formula that relates the numbers of hydrogens and carbons. The alkanes are CnH2n+2, the alkenes are CnH2n and the cycloalkanes are also CnH2n. The alkenes and the cycloalkanes are isomers. This means that they have the same molecular formula but different structural formula and thus may have completely different chemistry. Naming hydrocarbons follows the following route. Firstly name the longest chain. Then name any branches which come off and give the number of the carbon in the chain where they come off, e.g. When naming alkenes, the double bond should be numbered at the beginning of where it starts. Alkenes can undergo addition reactions, where small molecules can add across their double bond. It is common to add hydrogen or bromine in this way. Everyday Consumer Products: Carbohydrates contain carbon, hydrogen and oxygen and are often found in food stuffs. They can be burnt in the body by respiration to give out energy. We can test for carbon dioxide as it will turn lime water milky. Photosynthesis is the process by which plants store energy. Green pigments in the plant called chlorophyll are required to allow this to happen. Alcohol can be made by fermenting carbohydrates. Yeast is required for this process. Glucose + oxygen -> ethanol + carbon dioxide Enzymes are biological catalysts and they work best at specific temperatures and pHs. When an enzyme becomes too hot or the pH is unsuitable, then it becomes denatured (changes shape) and no longer works. The alcohols are a family of compounds that all contain the hydroxyl (-OH) group. They are named in a similar manner to alkanes. They can be made by hydrating (addition of water) alkenes. Ethanol is commonly known as drinking alcohol. Carboxylic acids all contain the carboxyl group (-COOH) and are acidic. They are named in a similar way to the alkanes and ethanonic acid is commonly known as vinegar. Esters are a group of compounds commonly known for their scent. They are used as flavourings and solvents. They are made by reacting alcohols and carboxylic acids together in a condensation reaction. And can be broken up to form these compounds by hydrolysis. Carbon compounds can be used as fuels, solvents, food, flavouring and many other things. Energy from Fuels: An exothermic reaction gives out heat (feels warm) and an endothermic reaction takes in heat (feels cold). Bond breaking requires energy (endothermic) and bond making gives out heat (exothermic). Fuel burning is an exothermic reaction and citric acid with baking powder is an endothermic reaction. The energy given out by a fuel can be calculated by: Eh (heat energy in kJ) = cmΔT c = specific heat capacity of water = 4.18kJ/kg⁰C m = mass of water in kg ΔT = change in temperature in ⁰C The equipment required for this procedure is: A metal beaker should be used to enable heat conduction and heat shields to stop heat being lost are also needed. This is a summary of what is in this topic – please look at your notes for further information.